Hyftor
Generic name: sirolimus
Dosage form: gel
Medically reviewed by A Ras MD. Last updated Apr 2, 2022
What is Hyftor
Hyftor Gel, 0.2%, is indicated for the treatment of angiofibroma associated with tuberous sclerosis in adults and children.
Hyftor Gel, 0.2% is not indicated for children younger than 3 years of age
Hyftor Dosage and Administration
For Topical Use Only.
- Apply a thin layer of Hyftor Gel, 0.2% (sirolimus) to the affected skin with angiofibroma twice daily. The minimum amount should be rubbed in gently and completely to control signs and symptoms of angiofibroma.
- Application should be limited to areas of involvement with angiofibroma’s.
The safety of Hyftor Gel, 0.2% under occlusion, which may promote systemic exposure, has not been evaluated. Hyftor Gel, 0.2% should not be used with occlusive dressings.
Dosage Forms and Strengths
Hyftor Gel, 0.2% comes in one strength: 0.2% (w/w%)
Hyftor Gel 0. 2% is for use on children aged 3 years and older and adults.
Contraindications
Hyftor Gel, 0.2% is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of the gel.
WARNINGS AND PRECAUTIONS.
Warnings
While a causal relationship has not been established, rare cases of skin malignancy and lymphoma have been reported in patients treated with oral mTOR inhibitors, including sirolimus.
Hyftor Gel, 0.2% should be administered as follows:
- Hyftor Gel, 0.2% application should be limited to areas of involvement with angiofibroma.
- Application sites should not be occluded.
- Hyftor Gel, 0.2% is not indicated for use in children less than 3 years of age.
- Hyftor Gel, 0.2% should not be used in immunocompromised adults and children.
- If symptoms do not improve within 12 weeks of treatment, reevaluate the proprietary for continuing Hyftor Gel, 0.2%. Hyftor Gel, 0.2% should only be continued with careful monitoring of symptoms.
- Patients should be cautioned to stay out of direct sunlight
Precautions
General
The use of Hyftor Gel, 0.2% may cause local symptoms such as irritation (dry skin, dermatitis, erythema, pruritus, acne). Localized symptoms are most common.
Bacterial and Viral Skin Infections
Before commencing treatment with Hyftor Gel, 0.2%, cutaneous bacterial or viral infections at treatment sites should be resolved. Studies have not evaluated the safety and efficacy of Hyftor Gel, 0.2% in the treatment of clinically infected angiofibroma.
Sun Exposure
During the course of treatment, patients should minimize or avoid natural or artificial sunlight exposure, even while Hyftor Gel, 0.2% is not on the skin. It is not known whether Hyftor Gel, 0.2% interferes with skin response to ultraviolet damage.
Immunocompromised Patients
The safety and efficacy of Hyftor Gel, 0.2% in immunocompromised patients have not been studied.
Adverse Reactions
Two 12-week randomized vehicle-controlled studies and one long-term safety study were conducted. 94 patients have been treated with Hyftor Gel, 0.2%.
The most common adverse events associated with Hyftor Gel, 0.2% application in the Phase 3 study were dry skin (36.7%), application site irritation (36.7%), pruritus (16.7%), and acne (6.7%).
The most common adverse events associated with Hyftor Gel, 0.2% application in the long term study were application site irritation (30.9%), dry skin (27.7%), acne (20.2%), pruritus and eye irritation (8.5%), erythema (7.4%), dermatitis acneiform (6.4%), and contact dermatitis (5.3%).
Reported adverse events were generally mild to moderate in severity and rarely led to treatment discontinuation.
The duration of long-term safety for adult and pediatric patients in the safety study is tabulated below.
| Time on Study | Adult | Pediatrics | Total |
| <52 weeks | 4 | 1 | 5 |
| ≥52 weeks, >104 weeks | 10 | 10 | 20 |
| ≥104 weeks | 30 | 39 | 69 |
The following table depicts the adjusted incidence of drug-related adverse events in the Phase 3 and the long-term study in the Hyftor Gel, 0.2% treatment groups.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
| System Organ Class (Preferred Term) | Phase 3 (N=30) No. of Patients (%) |
Long Term Study (N=94) No. of Patients (%) |
| Eye Disorders | ||
| Eye irritation | 1 (3.3) | 8 (8.5) |
| Skin and Subcutaneous Tissue Disorders | ||
| Acne | 2 (6.7) | 19 (20.2) |
| Dermatitis acneiform | 1 (3.3) | 6 (6.4) |
| Dermatitis contact | 0 (0.0) | 5 (5.3) |
| Dry skin | 11 (36.7) | 26 (27.7) |
| Erythema | 0 (0.0) | 7 (7.4) |
| Pruritus | 5 (16.7) | 8 (8.5) |
| General Disorders and Administration Site Conditions | ||
| Application site irritation | 11 (36.7) | 29 (30.9) |
Drug Interactions
Formal topical drug interaction studies with Hyftor Gel, 0.2% have not been conducted. Based on its extent of absorption, interactions of Hyftor Gel, 0.2% with systemically administered drugs are unlikely to occur but cannot be ruled out, The concomitant administration of strong CYP3A4 and P-gp inducers or strong CYP3A4/P-gp inhibitors that decrease or increase sirolimus concentrations should be avoided.
Ultraviolet phototherapies such as PUVA should be carefully performed because Hyftor Gel, 0.2% may develop the possibility of photosensitivity. Hyftor Gel, 0.2% has a potential activity in photosensitivity enhancement as observed in guinea pigs.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Hyftor Gel, 0.2% is not absorbed systemically following topical administration, and maternal use is not expected to result in fetal exposure of the drug.
Based on animal studies and the mechanism of action, sirolimus may cause fetal harm when administered orally to a pregnant woman. There are no adequate and well-controlled studies of topically administered sirolimus in pregnant women. The experience with Hyftor Gel, 0.2% when used by pregnant women is too limited to permit assessment of the safety of its use during pregnancy. However, in animal studies with oral sirolimus, it was embryo/fetotoxic in rats at sub-therapeutic doses. Advise pregnancy women of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the US general population, the estimate background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
No studies have been conducted with the topical route of administration.
In oral rat embryo-fetal development studies, pregnant rats were administered sirolimus orally during the period of organogenesis (Gestational Day 6-15). Sirolimus produced embryo-fetal lethality at 0.5 mg/kg (2.5-fold the clinical oral dose of 2 mg; based on body surface area) and reduced fetal weight at 1 mg/kg (5-fold the clinical oral dose of 2 mg, based on body surface area). The no observed adverse effect level (NOAEL) for fetal toxicity in rats was 0.1 mg/kg (0.5-fold the clinical oral dose of 2 mg, based on body surface area). Maternal toxicity (weight loss) was observed at 2 mg/kg (10-fold the clinical oral dose of 2 mg, based on body surface area). The NOAEL for maternal toxicity was 1 mg/kg. In combination with cyclosporine, rats had increased embryo-fetal mortality compared with sirolimus alone.
In oral rabbit embryo-fetal development studies, pregnant rabbits were administered sirolimus orally during the period of organogenesis (Gestational Day 6-18). There were no effects on embryo-fetal development at doses up to 0.05 mg/kg (0.5-fold the clinical oral dose of 2 mg, based on body surface area); however, at doses of 0.05 mg/kg and above, the ability to sustain a successful pregnancy was impaired (i.e., embryo-fetal abortion or early resorption). Maternal toxicity (decreased body weight) was observed at 0.05 mg/kg. The NOAEL for maternal toxicity was 0.025 mg/kg (0.25-fold the clinical oral dose of 2 mg, based on body surface area).
In an oral pre- and post-natal development study in rats, pregnant females were dosed during gestation and lactation (Gestational Day 6 through Lactation Day 20). An increased incidence of dead pups, resulting in reduced live litter size, occurred at 0.5 mg/kg (2.5-fold the clinical oral dose of 2 mg based on body surface area). At 0.1 mg/kg (0.5-fold the clinical oral dose of 2 mg, based on body surface area), there were no adverse effects on offspring. Sirolimus did not cause maternal toxicity or affect developmental parameters in the surviving offspring (morphological development, motor activity, learning, or fertility assessment) at 0.5 mg/kg, the highest dose tested.
Lactation
Risk Summary
Hyftor Gel, 0.2% is not absorbed systemically by the mother following topical administration, and breastfeeding is not expected to result in exposure of the child to Hyftor Gel, 0.2%. However, it is not known whether sirolimus is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from sirolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
After oral administration, sirolimus is present in the milk of lactating rats. There is potential for serious adverse effects from sirolimus in breastfed infants based on the mechanism of action.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Hyftor Gel, 0.2%, and any potential adverse effects on the breastfed child from Hyftor Gel, 0.2% or from the underlying maternal condition.
Females and Males of Reproductive Potential
Contraception
Females
Advise females of the potential for reprotoxicity as animal studies have shown that oral sirolimus can be harmful to the developing fetus. Females of reproductive potential are recommended to use highly effective contraceptive method during the administration of Hyftor Gel, 0.2%.
Males
Advise patients that the reproductive risk of Hyftor Gel, 0.2% is unknown.
Infertility
In fertility studies with oral sirolimus, changes in male rats included atrophic testes, epididymides, prostate, seminiferous tubules, and/or reduction in sperm counts, which resulted in reduced fertility. In females, reduced size of ovaries and uteri was observed. These changes observed in male and female rats occurred following oral administration of sirolimus at doses approximately 10 times or 2 times the clinical dose, respectively.
8.4 Pediatric Use
Hyftor Gel, 0.2% is not indicated for children less than 3 years of age.
Hyftor Gel, 0.2% has been studied in children 3 years and older.
The use of Hyftor Gel, 0.2% is not recommended in children below 3 years of age, since the safety and efficacy of Hyftor Gel, 0.2% have not been established in this age group.
The long-term safety and effects of Hyftor Gel, 0.2% on the developing immune system are unknown
Three studies were conducted involving a total of about 63 patients 3-18 years of age: two 12-week randomized vehicle-controlled studies and one open-label safety study conducted for up to two and a half years duration.
The most common adverse events associated with Hyftor Gel, 0.2% application in the long term safety study until 52 weeks (50 pediatric patients) were dry skin and application site irritation (26.0% each, 13 patients), pruritus (6.0%, 3 patients), and acne (12.0%, 6 patients). In the open-label safety studies, the incidence of adverse events, including infections, did not increase with the increased duration of study drug exposure or the amount of Gel used.
Geriatric Use
Studies of Hyftor Gel, 0.2% in geriatric patients have not been conducted.
Patients with Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of topically administered sirolimus has not been evaluated but dose-adjustment is not expected to be needed.
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of topically administered sirolimus has not been evaluated. However, there is minimal (2.3%) renal excretion of the drug or its metabolites in healthy volunteers following oral administration. On the basis of this information, dose-adjustment is not expected to be needed.
10 OVERDOSAGE
Hyftor Gel, 0.2% is not for oral use. Oral ingestion of Hyftor Gel, 0.2% may lead to adverse effects associated with systemic administration of sirolimus. If oral ingestion occurs, medical advice should be sought.
11 DESCRIPTION
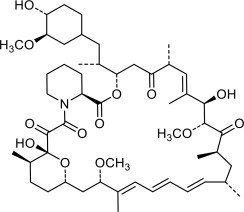
Hyftor Gel, 0.2% contains sirolimus, an mTOR inhibitor immunosuppressant. It is for topical dermatologic use only. Chemically, sirolimus is designated as (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34AS)-9,10,12,13, 14,21,22,23,24,25,26,27,32,33,34,34A-hexadecahydro-9,27-dihydroxy-3-((1R)-2-((1S,3R, 4R)-4-hydroxy-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido(2,1-C)-(1,4)oxaazacyclohentriacontine-1,5,11,28,29-(4H,6H,31H)-pentone. It has the following structural formula:
Sirolimus has a molecular formula of C51H79NO13 and a molecular weight of 914.17. Hyftor Gel, 0.2% contains sirolimus 2mg/g solubilized in a gel consisting of carboxyvinyl polymer, anhydrous ethanol, triethanolamine, and purified water.
Hyftor – Clinical Pharmacology
Mechanism of Action
The mechanism of action of sirolimus in the treatment of angiofibroma associated with tuberous sclerosis (TS) is unknown. TS is associated with genetic defects in TSC1 and TSC2 which leads to the constitutive activation of mammalian target of rapamycin (mTOR). Sirolimus inhibits mTOR activation by inhibition of phosphatidylinositol-3-kinase (PI3K)-like serine/threonine protein kinase. In cells, sirolimus binds to the immunophilin, FK Binding Protein-12 (FKBP-12), to generate an immunosuppressive complex. The sirolimus: FKBP-12 complex has no effect on calcineurin activity. This complex binds to and inhibits the activation of the mammalian Target of Rapamycin (mTOR), a key regulatory kinase. A germline mutation of the TSC1 or TSC2 gene, leading to activation of mTOR pathway, accounts for the pathogenesis of TSC-associated angiofibroma. Activated mTOR subsequently activates p70 ribosomal protein S6 kinase (p70S6K) and ribosomal protein S6 (S6) by phosphorylation (Chan et al., 2014). Sirolimus binds to the immunophilin, FK Binding Protein-12 (FKBP-12), to generate a complex. The sirolimus:FKBP-12 complex binds to and inhibits the activation of mTOR, a key regulatory kinase.
Pharmacokinetics
Absorption
Sirolimus blood concentrations ranged from undetectable to 0.50 ng/mL after multiple doses of 0.2% Hyftor Gel, 0.2% in the Phase 3 study, with 90% of the patients having blood concentrations less than 0.22 ng/mL. In the long-term study, periodic blood sampling demonstrated a similar distribution of sirolimus blood levels in pediatric and adult patients.
Mean peak sirolimus blood concentrations in healthy normal volunteers following oral administration (2 mg) was 5.5 ng/mL and could not be detected following topical administration of a dosage corresponding to 1.6 mg. The lowest sirolimus blood level at which systemic effects (e.g., immunosuppression) can be observed is not known.
Systemic levels of sirolimus have also been measured in pediatric patients
Special Populations: Pediatrics
The absorption of sirolimus was not increased in pediatric patients with TS treated with Hyftor Gel, 0.2%.
Distribution
There was no evidence based on blood concentrations that sirolimus accumulates systemically upon topical application in patients with TS for periods of up to 1 year.
Metabolism
Studies evaluating the metabolism of Hyftor Gel, 0.2% have not been conducted.
Sirolimus is a substrate for both CYP3A4 and P-gp. Sirolimus is extensively metabolized after oral administration in the intestinal wall and liver and undergoes counter-transport from enterocytes of the small intestine into the gut lumen. Inhibitors of CYP3A4 and P-gp increase sirolimus concentrations. Inducers of CYP3A4 and P-gp decrease sirolimus concentrations. Sirolimus is extensively metabolized by O-demethylation and/or hydroxylation. Seven (7) major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Sirolimus is the major component in human whole blood and contributes to more than 90% of the immunosuppressive activity.
Excretion
Studies evaluating the excretion of Hyftor Gel, 0.2% have not been conducted.
After a single dose of sirolimus oral solution in healthy volunteers, the majority (91%) of radioactivity was recovered from the feces, and only a minor amount (2.2%) was excreted in the urine. The mean ± SD terminal elimination half-life (t½) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 ± 16 hours.
Drug Interaction Studies
Formal topical drug interaction studies with Hyftor Gel, 0.2% have not been conducted. Based on its extent of absorption, interactions of Hyftor Gel, 0.2% with systemically administered drugs are unlikely to occur but cannot be ruled out. The concomitant administration of strong CYP3A4/P-gp inducers or strong CYP3A4/P-gp inhibitors that decrease or increase sirolimus concentrations should be avoided.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year dermal carcinogenicity studies have not been performed. Oral carcinogenicity studies were conducted in mice and rats. In an 86-week female mouse study at sirolimus doses 30 to 120 times higher than the 2 mg daily clinical dose (adjusted for body surface area), there was a statistically significant increase in malignant lymphoma at all dose levels compared with controls. In a second mouse study at dosages that were approximately 3 to 16 times the clinical dose (adjusted for body surface area), hepatocellular adenoma and carcinoma in males were considered sirolimus-related. In the 104-week rat study at dosages equal to or lower than the clinical dose of 2 mg daily (adjusted for body surface area), there were no significant findings.
Sirolimus was not genotoxic in the in vitro bacterial reverse mutation assay, the Chinese hamster ovary cell chromosomal aberration assay, the mouse lymphoma cell forward mutation assay, or the in vivo mouse micronucleus assay.
Reproductive toxicology studies were not performed with topical sirolimus gel. Fertility was diminished slightly in both male and female rats following oral administration of sirolimus at doses approximately 10 times or 2 times, respectively, the clinical dose of 2 mg daily (adjusted for body surface area). In male rats, atrophy of testes, epididymides, prostate, seminiferous tubules and/or reduction in sperm counts were observed. In female rats, ovarian and uterine atrophy was observed. Reduction of sperm count in male rats was reversible upon cessation of dosing in one study. Testicular tubular degeneration was also seen in a 4-week intravenous study of sirolimus in monkeys at doses that were approximately equal to the clinical dose (adjusted for body surface area)
Clinical Studies
A single randomized, double-blind, vehicle-controlled, multi-center, phase 3 study was conducted to evaluate Hyftor Gel, 0.2% for the treatment of patients with angiofibroma associated with TS. 28 men and 34 women enrolled in the study, with 27 of them 18 years of age and younger. In this study, patients applied either Hyftor Gel, 0.2% or vehicle gel twice daily to their face two times/day to 12 weeks.
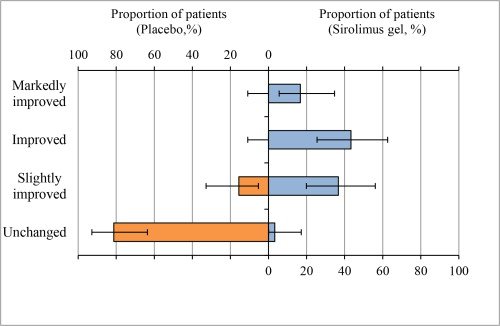
The primary outcome in this Phase 3 study was the composite improvement in size and redness of AF as assessed by Independent Review Committee using photographs at the end of Week 12. An assessment of ‘improved’ was defined as at least a 50% reduction in the size and a 2-level reduction in redness and an assessment of ‘markedly improved’ was defined as at least a 75% reduction in the size and a 3-level reduction in redness. A significantly greater (p < 0.001) percentage of patients achieved improvement in the composite of size and redness of angiofibroma at week 12 in the Hyftor Gel, 0.2% treatment groups compared to the vehicle treatment group. Improvement in the size and appearance of angiofibroma was appreciable as early as Week 4 with continued improvement during the treatment period (Week 12).
Primary Endpoint: Improvement in Angiofibroma at Week 12
In adult and pediatric Hyftor Gel, 0.2% patients, 60% were markedly improved or improved compared to 0% placebo-treated patients.
The investigator assessments were consistent with the primary endpoint.
How Supplied/Storage and Handling
HyftorTM (sirolimus) Gel, 0.2%
Store at refrigerated at 2-8°C (36-46°F).
Distributed by:
Nobelpharma America, LLC
Patient Counseling Information
Advise patients, their families, and their caregivers to read the Medication Guide and assist them in understanding its contents. The complete text of the Medication Guide is reprinted at the end of the document.¶
Information for Patients
Patients using Hyftor Gel, 0.2% should receive and understand the information in the Medication Guide. Please refer to the Medication Guide for providing instruction and information to the patient.
What is the most important information patients should know about Hyftor Gel, 0.2%?
The safety of using Hyftor Gel, 0.2% for a long period of time is not known. A very small number of people who have used oral sirolimus have had cancer (for example, skin or lymphoma). However, a link with Hyftor Gel,0.2% has not been shown. Because of this concern, instruct patients:
- Use Hyftor Gel, 0.2% only on areas of skin that have angiofibroma. Do not use Hyftor Gel, 0.2% on children under 3 years old.
Hyftor Gel, 0.2% comes in one strength: 0.2% (w/w%)
- Hyftor Gel, 0. 2% is for use on children aged 3 years and older and adults.
Advise patients to talk to their prescriber for more information.
How should Hyftor Gel, 0.2% be used?
Advise patients to:
- Use Hyftor Gel, 0.2% exactly as prescribed.
- Use Hyftor Gel, 0.2% only on areas of skin that have angiofibroma.
- Follow their doctor’s advice if symptoms of angiofibroma return after treatment with Hyftor Gel, 0.2%.
- Call their doctor if:
- Their symptoms get worse with Hyftor Gel, 0.2%.
- They get an infection on their skin.
To apply Hyftor Gel, 0.2%:
Advise patients:
- Wash their hands before applying Hyftor Gel, 0.2%.
- Apply a thin layer of Hyftor Gel, 0.2% twice daily to the areas of skin affected by angiofibroma.
- Use the smallest amount of Hyftor Gel, 0.2% needed to control the angiofibroma.
- If they are a patient or a caregiver applying Hyftor Gel, 0.2% to a patient wash their hands with soap and water after applying Hyftor Gel, 0.2%. This should remove any Hyftor Gel, 0.2% left on the hands.
- Do not bathe, shower, or swim right after applying HYLFORTM Gel. This could wash off the gel.
What should patients avoid while using Hyftor Gel, 0.2%?
Advise patients:
- Do not use ultraviolet light therapy, sun lamps, or tanning beds during treatment with Hyftor Gel, 0.2%.
- Limit sun exposure during treatment with Hyftor Gel, 0.2% even when the medicine has not been applied to the skin. Doctors should advise what other types of protection from the sun patients should use.
- Do not cover the skin being treated with bandages, dressings or wraps.
- Avoid getting Hyftor Gel, 0.2% in the eyes or mouth. Do not swallow Hyftor Gel, 0.2%.
- Potential risk to a fetus
MEDICATION GUIDE
Hyftor Gel, 0.2%
(sirolimus)
Read the Medication Guide every time you or a family member receives Hyftor Gel, 0.2%. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. If you have questions about Hyftor Gel, 0.2%, ask your doctor or pharmacist.
What is the most important information I should know about Hyftor Gel, 0.2%?
The safety of using Hyftor Gel, 0.2% for a long period of time is not known.
Because of this concern:
- Use Hyftor Gel, 0.2% only on areas of your skin that have angiofibroma.
- Do not use Hyftor Gel, 0.2% on a child under 3 years old.
Hyftor Gel, 0.2% is:
- for use on children aged 3 years and adults.
Talk to your doctor for more information.
What is Hyftor Gel, 0.2%?
Hyftor Gel, 0.2% is a prescription medicine used on the skin (topical) applied to angiofibroma. Hyftor Gel, 0.2% is in a class of medicines called topical mTOR inhibitors. It is for adults and children 3 years of age and older
Who should not use Hyftor Gel, 0.2%?
Hyftor Gel, 0.2% should not be used:
- on children younger than 3 years of age.
- if you are allergic to Hyftor Gel, 0.2% or its ingredients. See the end of this Medication Guide for a complete list of ingredients.
- In pregnant women
- Applied to bleeding or ulcerated tumors
What should I tell my doctor before starting Hyftor Gel, 0.2%?
Before you start using Hyftor Gel, 0.2%, you and your doctor should talk about all of your medical conditions, including if you:
- have any infection, or itching on your skin.
- have been told you have a weakened immune system or have cancer.
- are pregnant, breastfeeding, or planning to become pregnant.
Tell your doctor about all the medicines you take and skin products you use including prescription and nonprescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I use Hyftor Gel, 0.2%?
- Use Hyftor Gel, 0.2% exactly as prescribed.
- Use Hyftor Gel, 0.2% only on areas of your skin that have angiofibroma.
- Call your doctor if:
- your symptoms get worse with Hyftor Gel, 0.2%.
- you get an infection on your skin.
To apply Hyftor:
- Wash your hands before applying Hyftor Gel, 0.2%.
- Apply a thin layer of Hyftor Gel, 0.2% twice daily to angiofibroma affected areas.
- Use the smallest amount of Hyftor Gel, 0.2% to cover the angiofibroma.
- If you are a patient or caregiver applying Hyftor Gel. 0.2% to a patient, , wash your hands with soap and water after applying Hyftor Gel, 0.2%. This should remove any gel left on the hands.
- Do not bathe, shower, or swim immediately after applying Hyftor Gel, 0.2% as this could wash off the gel.
- You can use sunscreen and moisturizers with Hyftor Gel, 0.2%. Make sure you check with your doctor first about the products that are right for you. If you use sunscreen or moisturizers, apply them after Hyftor Gel, 0.2%.
What should I avoid while using Hyftor Gel, 0.2%?
- Do not use ultraviolet light therapy, sun lamps, or tanning beds during treatment with Hyftor Gel, 0.2%.
- Limit sun exposure during treatment with Hyftor Gel, 0.2%. Ask your doctor what types of protection from the sun you should use.
- Do not cover the skin being treated with bandages, dressings or wraps.
- Avoid getting Hyftor Gel, 0.2% in the eyes or mouth. Do not swallow Hyftor Gel, 0.2.
What are the possible side effects of Hyftor Gel, 0.2%?
Please read the first section of this Medication Guide.
The most common side effects of Hyftor Gel, 0.2% at the skin application site are dermal irritation of the skin.
Other side effects.
Talk to your doctor if you have a skin infection or if side effects continue or bother you.
These are not all the side effects with Hyftor Gel, 0.2%. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
How should I store Hyftor Gel, 0.2%?
- Store Hyftor Gel, 0.2% under refrigeration at 36-46°F (2-8°C).
- Do not leave Hyftor Gel, 0.2% out of refrigeration, greater than 46°F (8°C).
- Do not leave Hyftor Gel 0.2% at extreme temperatures, above 104°F(40°C).
- Keep Hyftor Gel, 0.2% away from an open flame.
- Make sure the cap on the tube is tightly closed after each use.
- Keep Hyftor Gel, 0.2% and all medicines out of the reach of children.
General advice about Hyftor Gel
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Hyftor Gel, 0.2% for a condition for which it was not prescribed. Do not give Hyftor Gel to other people, even if they have the same symptoms you have. It may not be right for them.
This Medication Guide summarizes the most important information about Hyftor Gel, 0.2%. If you would like more information, talk with your doctor.
Your doctor or pharmacist can give you information about Hyftor Gel, 0.2% that is written for health care professionals. For more information, you can also visit the Hyftor website at www.Hyftor.com or call 887-375-0825
What are the ingredients in Hyftor Gel, 0.2%?
Active Ingredient: sirolimus, 0.2%
Inactive Ingredients: carboxyvinyl polymer, anhydrous ethanol, triethanolamine, and purified water
Distributed by:
Nobelpharma America, LLC
This Medication Guide has been approved by the U.S. Food and Drug Administration
Principal Display Panel – Carton Label
NDC 73683-101-10 Rx Only
Hyftor
Sirolimus gel
0.2%
For topical use only
Keep refrigerated
10 g tube
Nobelpharma
Principal Display Panel – Tube Label
NDC 73683-101-10 Rx Only
Hyftor
Sirolimus gel
0.2%
For topical use only
Keep refrigerated
10 g tube
Nobelpharma