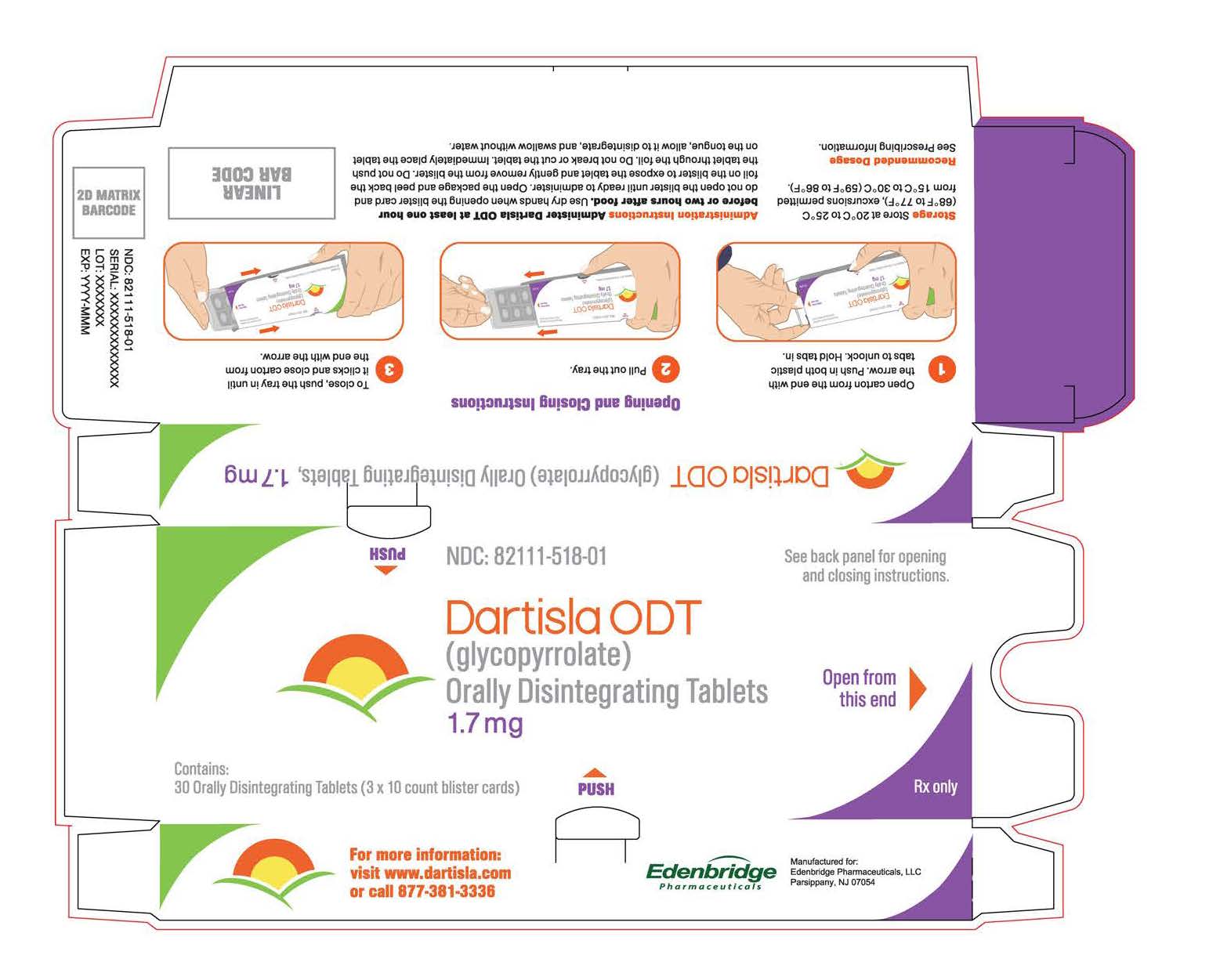
Dartisla ODT
Generic name: glycopyrrolate
Dosage form: orally disintegrating tablets tablets
Drug class: Anticholinergics / antispasmodics
Medically reviewed by A Ras MD. Last updated April 13, 2022
What is Dartisla ODT
Dartisla ODT is a prescription drug used to treat the symptoms of Neuromuscular Blockade Reversal, Adjunct to Peptic Ulcer Treatment, and preoperative or intraoperative cholinergic effects decrease during surgery. Dartisla ODT can be taken on its alone or in combination with other drugs.
Dartisla ODT belongs to the Anesthetic Premedication Agents; Anticholinergic Agents class of medicines.
Dartisla ODT is not known to be safe or effective in children under the age of one month.
1 INDICATIONS & USAGE
 DARTISLA ODT is indicated in adults to reduce symptoms of a peptic ulcer as an adjunct to treatment of peptic ulcer.
DARTISLA ODT is indicated in adults to reduce symptoms of a peptic ulcer as an adjunct to treatment of peptic ulcer.
Limitations of Use
DARTISLA ODT is not indicated as monotherapy for treatment of peptic ulcer because effectiveness in peptic ulcer healing has not been established.¶
2 DOSAGE & ADMINISTRATION
2.1 Important Dosing Information
-
- Patients receiving the 2 mg dosage strength of another oral tablet dosage form of glycopyrrolate may be switched to the 1.7 mg dosage strength of DARTISLA ODT [see Dosage Forms and Strengths (3) and Clinical Pharmacology (12.3)].
-
- DARTISLA ODT is not recommended for patients in whom a lower dosage strength of another oral glycopyrrolate product (e.g., tablet strength of 1 mg) is appropriate for initial or maintenance treatment because the dosage strength of DARTISLA ODT may exceed the recommended initial and maintenance dosage of other oral glycopyrrolate products.
2.2 Recommended Dosage
- The recommended dosage of DARTISLA ODT is 1.7 mg given two or three times daily administered on top of the tongue. Allow the tablet to disintegrate and swallow without water [see Clinical Pharmacology (12.3)].
- The maximum recommended daily dosage of DARTISLA ODT is 6.8 mg.
- Administer DARTISLA ODT at least one hour before or two hours after food [see Clinical Pharmacology (12.3)].
- Use the lowest effective dosage of glycopyrrolate to control symptoms. Switch patients who can be titrated to a lower dose of oral glycopyrrolate to another oral tablet dosage form of glycopyrrolate.
2.3 Administration Instructions
-
- Use dry hands when opening the blister card and do not open the blister until ready to administer.
- Open the package and peel back the foil on the blister to expose the tablet and gently remove from the blister. Do not push the tablet through the foil.
- Do not break or cut the tablet.
- Immediately place the tablet on the tongue, allow it to disintegrate, and swallow without water.
3 DOSAGE FORMS & STRENGTHS
Orally Disintegrating Tablets: 1.7 mg of glycopyrrolate, white to off-white, round, and debossed with the symbol  .
.
4 CONTRAINDICATIONS(WHAT IS THIS?)
DARTISLA ODT is contraindicated in:
- Patients at risk for anticholinergic toxicity due to an underlying medical condition, including:
- Glaucoma [see Warnings and Precautions (5.1)]
- Obstructive uropathies including prostatic hypertrophy
- Mechanical obstructive diseases of gastrointestinal tract (e.g., pyloroduodenal stenosis, strictures) [see Warnings and Precautions (5.2)]
- Gastrointestinal motility disorders (e.g., achalasia, paralytic ileus, intestinal atony) [see Warnings and Precautions (5.3)]
- Bleeding gastrointestinal ulcer
- Active inflammatory or infectious colitis which can lead to toxic megacolon
- History of or current toxic megacolon
- Myasthenia gravis
- Patients with a hypersensitivity to glycopyrrolate or any of the inactive ingredients in DARTISLA ODT [see Adverse Reactions (6) and Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Precipitation of Acute Glaucoma
Glycopyrrolate may cause increased intraocular pressure in patients with glaucoma and reduce the effects of antiglaucoma agents. Instruct patients to discontinue DARTISLA ODT and promptly seek medical care if they experience symptoms of acute angle closure glaucoma (pain and reddening of the eyes accompanied by dilated pupils) [see Contraindications (4)]
5.2 Partial or Complete Mechanical Intestinal Obstruction
DARTISLA ODT may worsen intestinal mechanical obstruction, and diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. If partial or complete intestinal obstruction is suspected, discontinue use of DARTISLA ODT and evaluate for potential intestinal obstruction [see Contraindications (4)].
5.3 Gastrointestinal Adverse Reactions Due to Decreased Gastrointestinal Motility
Glycopyrrolate reduces gastrointestinal motility and may result in delayed gastric emptying, constipation, and intestinal pseudo-obstruction and may precipitate or aggravate paralytic ileus and toxic megacolon [see Contraindications (4)]. The risk of gastrointestinal adverse reactions is further increased with use of other anticholinergics and other medications that decrease gastrointestinal peristalsis. Monitor patients for symptoms of decreased gastrointestinal motility. Concomitant use of DARTISLA ODT and other anticholinergics or other medications that decrease GI peristalsis is not recommended [see Drug Interactions (7.2)].
5.4 Cognitive and Visual Adverse Reactions
Glycopyrrolate may produce drowsiness and blurred vision and impair the mental and/or physical abilities required for the performance of hazardous tasks such as driving a motor vehicle, operating machinery or performing other hazardous work [see Adverse Reactions (6)]. Concomitant use of other drugs that have anticholinergic properties may increase these effects [see Drug Interactions (7.1)]. Inform patients not to operate motor vehicles or other dangerous machinery or perform other hazardous tasks until they are reasonably certain that DARTISLA ODT does not affect them adversely. Discontinue DARTISLA ODT if signs or symptoms of cognitive or visual impairment develop.
5.5 Heat Prostration at High Environmental Temperatures
In the presence of a high environmental temperature, heat prostration resulting in fever and heat stroke can occur with use of DARTISLA ODT due to decreased sweating, particularly in geriatric patients [see Adverse Reactions (6)]. Advise patients to avoid exposure to hot or very warm environmental temperatures when taking DARTISLA ODT. DARTISLA ODT is not recommended in geriatric patients [see Warnings and Precautions (5.7)].
5.6 Other Conditions Exacerbated by Anticholinergic Adverse Reactions
DARTISLA ODT is not recommended in patients with other conditions exacerbated by anticholinergic adverse reactions (e.g., autonomic neuropathy, hyperthyroidism, cardiac disease, and hiatal hernia associated with reflux esophagitis) and in patients taking other anticholinergic medications [see Drug Interactions (7.1)].
5.7 Increased Risk of Anticholinergic Adverse Reactions in Geriatric Patients
Geriatric patients 65 years of age and older are at increased risk of anticholinergic adverse reactions that may lead to complications of urinary retention, bowel obstruction, heat prostration, arrhythmias, delirium, and falls or fractures. DARTISLA ODT is not recommended in geriatric patients and may be contraindicated in some geriatric patients with underlying medical conditions [see Contraindications (4), Warnings and Precautions (5.2, 5.5), Adverse Reactions (6) and Use in Specific Populations (8.5)].
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are discussed elsewhere in the labeling:
- Precipitation of Acute Glaucoma [see Warnings and Precautions (5.1)]
- Partial or Complete Mechanical Intestinal Obstruction [see Warnings and Precautions (5.2)]
- Gastrointestinal Adverse Reactions due to Decreased Gastrointestinal Motility [see Warnings and Precautions (5.3)]
- Cognitive and Visual Adverse Reactions [see Warnings and Precautions (5.4)]
- Heat Prostration at High Environmental Temperatures [see Warnings and Precautions (5.5)]
- Other Conditions Exacerbated by Anticholinergic Adverse Reactions [see Warnings and Precautions (5.6)]
- Increased Risk of Anticholinergic Adverse Reactions in Geriatric Patients [see Warnings and Precautions (5.7)]
The following adverse reactions associated with the use of glycopyrrolate, or other anticholinergic drugs, were identified in clinical studies or postmarketing reports. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: chest pain, hypertension, tachycardia
Endocrine Disorders: decreased sweating
Eye Disorders: blurred vision, cycloplegia, dilatation of the pupil, increased ocular tension
Gastrointestinal Disorders: bloated feeling, constipation, dry mouth, dysgeusia, nausea, vomiting
Immune System Disorders: anaphylaxis [see Contraindications (4)]
Nervous System Disorders: agitation, dizziness, drowsiness, headache, insomnia, mental confusion, nervousness, weakness
Respiratory Disorders: respiratory depression, throat irritation
Renal and Urinary Disorders: urinary hesitancy, urinary retention
Reproductive System and Breast Disorders: impotence, suppression of lactation
Vascular Disorders: flushing
7 DRUG INTERACTIONS
7.1 Other Anticholinergic Drugs
There is potential for an additive interaction between glycopyrrolate and concomitantly used anticholinergic drugs (e.g., tricyclic antidepressants, anti-epileptics, class I antiarrhythmics, anti-spasmodics, amantadine) resulting in increased anticholinergic adverse reactions. Coadministration of antipsychotics with glycopyrrolate may lead to worsening of tardive dyskinesia. DARTISLA ODT is not recommended in patients taking other anticholinergic drugs [see Warnings and Precautions (5.3, 5.4, 5.6)].
7.2 Drugs with Altered Absorption due to Decreased Gastrointestinal Motility and Increased Transit Time
Decreased gastrointestinal motility by glycopyrrolate may impact absorption of other drugs leading to increased or decreased drug exposure. DARTISLA ODT is not recommended in patients taking other drugs that are affected by altered gastrointestinal motility. [see Warnings and Precautions (5.3)].
7.3 Gastrointestinal Toxicity with Solid Oral Dosage Forms of Potassium Chloride
Oral glycopyrrolate may worsen gastrointestinal mucosal injury reported with solid oral dosage forms of potassium chloride due to decreased gastric motility and increased transit time leading to prolonged contact with the gastrointestinal mucosa. DARTISLA ODT is not recommended in patients taking solid oral dosage forms of potassium chloride.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Over decades of use, there is an absence of published data on orally administered glycopyrrolate in pregnant women, including an absence of any reports of a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal studies, at non-maternally toxic doses of oral glycopyrrolate, there were no adverse developmental effects in rats or rabbits. A pre- and post-natal development study of oral glycopyrrolate in rats showed a decrease in pup mean body weight that recovered post nursing, with no other developmental effects observed (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
At non-maternally toxic doses of oral glycopyrrolate there were no effects on embryo-fetal development or toxicity in rats or rabbits. A pre- and post-natal development study of oral glycopyrrolate in rats showed a decrease in pup mean body weight that recovered post nursing, with no other developmental effects observed.
In a published reproductive and developmental study, male and female rats were administered glycopyrrolate in the diet at 0, 32.5, 63 and 130 mg/kg/day for 3 to 5 weeks and through up to three consecutive litters. There was no indication of abnormalities in the pups of treated dams. There was a decreased rate of conception and in survival rate at weaning for all treated animals in a dose-related manner. Diminished rates of conception may be due to diminished seminal secretion [see Nonclinical Toxicology (13.1)].
8.2 Lactation
Risk Summary
There are no data on the presence of glycopyrrolate in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. As with other anticholinergic drugs, glycopyrrolate may cause suppression of lactation.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DARTISLA ODT and any potential adverse effects on the breastfed infant from DARTISLA ODT.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Geriatric patients 65 years of age and older may be more sensitive to the anticholinergic adverse reactions of glycopyrrolate leading to complications of urinary retention, bowel obstruction, heat prostration, arrhythmias, delirium, and falls or fractures;therefore, DARTISLA ODT is not recommended in geriatric patients and may be contraindicated in some geriatric patients with underlying medical conditions [see Contraindications (4) and Warnings and Precautions (5)].
8.6 Renal Impairment
Glycopyrrolate is substantially excreted by the kidney [see Clinical Pharmacology (12.3)]. Monitor patients with renal impairment for anticholinergic adverse reactions [see Adverse Reactions (6)].If anticholinergic adverse reactions occur, discontinue DARTISLA ODT.
10 OVERDOSAGE
Signs and symptoms of glycopyrrolate overdosage are related to excessive anti-muscarinic anticholinergic activity and are generally peripheral (e.g., flushing, hyperthermia, tachycardia, ileus, urinary retention, loss of ocular accommodation and light sensitivity due to mydriasis), but central nervous system toxicity (agitation, seizures, hyperthermia) may also occur.
If over-exposure occurs, call the Poison Control Center at 1-800-222-1222 for current information on the management of glycopyrrolate poisoning and overdosage.
Management of glycopyrrolate overdosage is based upon presenting signs and symptoms, including close observation for severe or life-threatening complications which may require respiratory and cardiovascular monitoring and support. Consider administration of activated charcoal and/or use of a reversible anticholinesterase as appropriate or recommended by Poison Control.
11 DESCRIPTION
DARTISLA ODT (glycopyrrolate) orally disintegrating tablets contains the synthetic anticholinergic, glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name: 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide. The empirical formula for glycopyrrolate is C19H28BrNO3, the molecular weight is 398.3 g/mol, and the structural formula is:
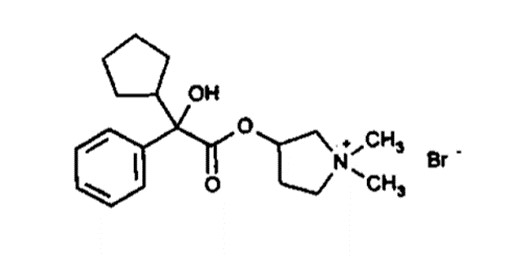
Each DARTISLA ODT contains: glycopyrrolate, USP 1.7 mg as the active ingredient. The inactive ingredients include black cherry, citric acid, fish gelatin (high molecular weight), mannitol, poloxamer 188, purified water and sucralose micronized.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glycopyrrolate, an anticholinergic (antimuscarinic) agent, inhibits the action of acetylcholine on parietal cells in the stomach and decreases the volume and acidity of gastric secretions.
12.2 Pharmacodynamics
No formal pharmacodynamic studies have been conducted with DARTISLA ODT.
12.3 Pharmacokinetics
Absorption
After DARTISLA ODT administration under fasted conditions, the mean (SD) Cmax was 390 (±237) pg/mL, and the mean AUC0-t and AUC0-inf was 1862 (±1116) and 1977 (± 1171) pg·hr/mL, respectively. The median time to maximum plasma concentration was 3 hours. After DARTISLA ODT 1.7 mg administration, the Cmax and AUC of glycopyrrolate were comparable to an oral 2 mg glycopyrrolate tablet [see Dosage and Administration (2.2)].
When DARTISLA ODT was placed in the mouth and immediately swallowed with 240 mL water, the mean Cmax and AUC of glycopyrrolate decreased by 24% and 20%, respectively, compared to administration without water [see Dosage and Administration (2.2)].
Effect of Food
In healthy adults, a high-fat, high-calorie meal (939 calories, 60% fat) significantly reduced the absorption of glycopyrrolate following administration of DARTISLA ODT 1.7 mg. The mean Cmax and AUC were approximately 83% and 77% lower, respectively, than those observed under fasted conditions [see Dosage and Administration (2.2)].
Elimination
After DARTISLA ODT 1.7 mg administration, the mean plasma half-life was 2.8 hours.
Specific Populations
Patients with Renal Impairment
In published literature, glycopyrrolate 4 mcg/kg was administered intravenously (DARTISLA ODT is not recommended for intravenous use) in uremic patients undergoing renal transplantation surgery. The mean AUC (10.6 mcg·h/L), and 24-hour urinary excretion (7%) for glycopyrrolate were significantly different from normal healthy adult subjects undergoing general surgery (3.7 mcg·h/L, and 65%, respectively) [see Use in Specific Populations (8.6)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Reproduction studies in rats resulted in diminished rates of conception, in a dose-related manner. Studies in dogs suggest that diminished rates of conception may be due to diminished seminal secretion, which is evident at high doses of glycopyrrolate.
16 HOW SUPPLIED/STORAGE AND HANDLING
DARTISLA ODT is supplied as 1.7 mg glycopyrrolate as a white to off-white, round orally disintegrating tablets debossed with the symbol  . Available as:
. Available as:
- NDC 82111-518-01: 30 orally disintegrating tablets per carton. Each carton has 3 blister cards containing 10 orally disintegrating tablets each.
Store at 20°C to 25°C (68°F to 77°F); with excursions between 15°C to 30°C (59°F to 86°F) [USP controlled room temperature].
17 PATIENT COUNSELING INFORMATION
Precipitation of Acute Glaucoma
Advise patient to discontinue DARTISLA ODT and promptly seek medical care if they experience symptoms of acute angle closure glaucoma (pain and reddening of the eyes accompanied by dilated pupils) [see Warnings and Precautions (5.1)].
Partial or Complete Mechanical Intestinal Obstruction
Advise patients to contact their healthcare provider if diarrhea occurs, especially in patients with ileostomy or colostomy [see Warnings and Precautions (5.2)].
Gastrointestinal Adverse Reactions Due to Decreased Gastrointestinal Motility
Inform patients that DARTISLA ODT may cause adverse reactions related to decreased gastrointestinal motility and to report to their healthcare provider if they experience symptoms such as vomiting, early satiety, abdominal distention, and constipation [see Warnings and Precautions (5.3)].
Cognitive and Visual Adverse Reactions
Inform patients that DARTISLA ODT may cause cognitive or visual impairment and not to operate motor vehicles or other dangerous machinery or perform other hazardous tasks until they are reasonably certain that DARTISLA ODT does not affect them adversely. Advise patients to discontinue DARTISLA ODT immediately and contact their healthcare provider if symptoms develop (e.g., drowsiness or blurred vision) [see Warnings and Precautions (5.4)].
Heat Prostration at High Environmental Temperatures
Inform patients that DARTISLA ODT can reduce sweating, leading to the possibility of heat exhaustion or heat stroke. Advise patients to avoid exposure to hot or very warm environmental temperatures [see Warnings and Precautions (5.5)].
Dosage and Administration Instructions
- Administer DARTISLA ODT at least one hour before or two hours after food [see Dosage and Administration (2.2)].
- Use dry hands when handling the blister card and do not open the blister until ready to administer.
- Open the package and peel back the foil on the blister to expose the tablet and gently remove from the blister. Do not push the tablet through the foil.
- Do not break or cut the tablet.
- Immediately place the tablet on the tongue, allow it to disintegrate, and swallow without water [see Dosage and Administration (2.3)].
Manufactured by:
Catalent Pharma Solutions Limited
Frankland Road
Blagrove, Swindon
Wiltshire SN5 8RU, United Kingdom (GBR)
Manufactured for:
Edenbridge Pharmaceuticals, LLC
Parsippany, NJ 07054

© 2021 Edenbridge Pharmaceuticals, LLC
Label
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL(WHAT IS THIS?)