Hyftor
Generic name: sirolimus
Dosage form: topical gel
Drug classes: MTOR inhibitors, Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Hyftor?
Hyftor is a prescription medicine that is used on the skin (topical) to treat adults and children 6 years of age and older with a type of noncancerous tumor called angiofibroma on your face caused by the genetic condition tuberous sclerosis.
It is not known if Hyftor is safe and effective in children under 6 years of age.
Description
HYFTOR™ (sirolimus topical gel) 0.2% is an mTOR inhibitor immunosuppressant for topical use. Each gram contains 2 mg of sirolimus, which is solubilized in a gel consisting of alcohol 51%, Carbomer 940, purified water, and trolamine.
Chemically, sirolimus is designated as (3 S,6 R,7 E,9 R,10 R,12 R,14 S,15 E,17 E,19 E,21 S,23 S,26 R,27 R,34a S)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-[(1 R)-2-[(1 S,3 R,4 R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3 H-pyrido[2,1- c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4 H,6 H,31 H)-pentone. It has the following structural formula:
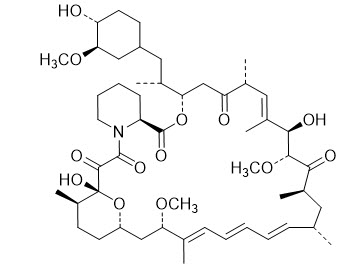
Sirolimus is a white to off-white powder and is insoluble in water, but freely soluble in chloroform, acetone and acetonitrile. Sirolimus has a molecular formula of C 51H 79NO 13 and a molecular weight of 914.19.
Mechanism of Action
The mechanism of action of sirolimus in the treatment of angiofibroma associated with tuberous sclerosis is unknown. Tuberous sclerosis is associated with genetic defects in TSC1 and TSC2 which leads to the constitutive activation of mammalian target of rapamycin (mTOR). Sirolimus inhibits mTOR activation.
What is the most important information I should know about Hyftor?
Hyftor is for use on the skin only (topical use). Do not use Hyftor in your mouth, eyes, or vagina.
Who should not use Hyftor?
Do not use Hyftor if you are allergic to sirolimus or any of the other ingredients in Hyftor. See the end of this leaflet for a complete list of ingredients in Hyftor.
What should I tell my healthcare provider before using Hyftor?
Before using Hyftor, tell your healthcare provider about all of your medical conditions, including if you:
- have a skin infection at the treatment site
- have high cholesterol or high triglycerides (fat or lipids) in your blood
- are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with Hyftor. Vaccines may be less effective during treatment with Hyftor.
- are pregnant or plan to become pregnant. Hyftor may harm your unborn baby. You should not become pregnant during treatment with Hyftor.
- Females who are able to become pregnant should use effective birth control (contraception) before starting treatment with Hyftor, during treatment, and for 12 weeks after your final dose of Hyftor. Talk to your healthcare provider about types of birth control that you can use during this time.
- are breastfeeding or plan to breastfeed. It is not known if Hyftor passes into your breast milk. You should not breastfeed during treatment with Hyftor.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using Hyftor with certain medicines may affect each other causing side effects.
How should I use Hyftor?
- Use Hyftor exactly as your healthcare provider tells you to use it.
- Before you use Hyftor, your healthcare provider or pharmacist should show you how to correctly measure your dose.
- Wash your hands before and after applying Hyftor.
- Apply Hyftor to the skin of the face affected with angiofibroma 2 times a day, in the morning and at bedtime.
- Do not cover, wrap, apply dressings, or bandage the skin area treated with Hyftor.
- Tell your healthcare provider if the treated skin area does not improve within 12 weeks of treatment.
What should I avoid while using Hyftor?
Limit your exposure to sunlight and ultraviolet light, such as tanning beds and ultraviolet light therapy, during treatment with Hyftor. Wear clothing that covers your skin if you need to go outside. Talk with your healthcare provider about other ways you can protect your skin from the sun.
What are the possible side effects of Hyftor?
Hyftor may cause serious side effects, including:
- Allergic reactions. Serious allergic reactions have happened in people who have taken sirolimus by mouth. Stop using Hyftor and get medical help right away if you get any of these symptoms of a serious allergic reaction:
- swelling of your face, eyes, or mouth
- trouble breathing or wheezing
- throat tightness
- chest pain or tightness
- feeling dizzy or faint
- rash or peeling of your skin
- Infections. Serious infections, including infections that can happen when your immune system is weak, have happened in people who have taken sirolimus by mouth. Some people have developed a rare, serious brain infection called progressive multifocal leukoencephalopathy (PML) which can sometimes cause death. Stop using Hyftor and call your healthcare provider right away if you get symptoms of an infection including fever or chills.
- Risk of cancer. Lymphoma and other cancers, especially skin cancer, have happened in people who have taken sirolimus by mouth. Talk with your healthcare provider about your risk for cancer if you use Hyftor.
- Increased levels of cholesterol and triglycerides (fat or lipids) in the blood have happened in people who have taken sirolimus by mouth . Your healthcare provider may do blood tests to check you for high lipid levels during treatment with Hyftor and treat you, if needed.
- Lung or breathing problems. Lung or breathing problems, including problems that have sometimes caused death, have happened in people who have taken sirolimus by mouth. Stop using Hyftor and get medical help right away if you get symptoms such as shortness of breath, new or worsening cough, or chest pain.
The most common side effects of Hyftor include dry skin, application site irritation, itching, acne, acne-like rash, eye redness, skin bleeding, and skin irritation.
Hyftor may cause fertility problems in males and females, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of Hyftor.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Hyftor
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Hyftor for a condition for which it was not prescribed. Do not give Hyftor to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Hyftor that is written for health professionals.
How should I store Hyftor?
- Store Hyftor in the refrigerator between at 36°F to 46°F (2°C to 8°C).
- Keep Hyftor out of light.
Keep Hyftor and all medicines out of the reach of children.
What are the ingredients in Hyftor?
Active ingredient: sirolimus
Inactive ingredients: alcohol 51%, Carbomer 940, purified water, and trolamine.
Label
PRINCIPAL DISPLAY PANEL – 10 G TUBE CARTON
- NDC 73683-101-10
- HYFTOR™
(sirolimus topical gel) 0.2% - Nobel pharma
- For topical use only | Keep refrigerated | 10 g tube | Rx Only
- Dispense enclosed Patient Information to patient

SRC: NLM .