Injectafer
Generic name: ferric carboxymaltose
Drug class: Iron products
Medically reviewed by A Ras MD.
What is Injectafer?
Injectafer is a prescription iron replacement medicine used to treat iron deficiency anemia in adults who have intolerance to oral iron or who have not responded well to treatment with oral iron non-dialysis dependent chronic kidney disease
It is not known if Injectafer is safe and effective for use in children.
Description
Ferric carboxymaltose, an iron replacement product, is an iron carbohydrate complex with the chemical name of polynuclear iron (III)-hydroxide 4(R)-(poly-(1→4)-O-α-D-glucopyranosyl)-oxy-2(R),3(R),5(R),6-tetrahydroxy-hexanoate. It has a relative molecular weight of approximately 150,000 Da corresponding to the following empirical formula:
[FeOx(OH)y(H2O)z]n [{(C6H10O5)m (C6H12O7)}l]k,
where n ≈ 103, m ≈ 8, l ≈ 11, and k ≈ 4
(l represents the mean branching degree of the ligand).
The chemical structure is presented below: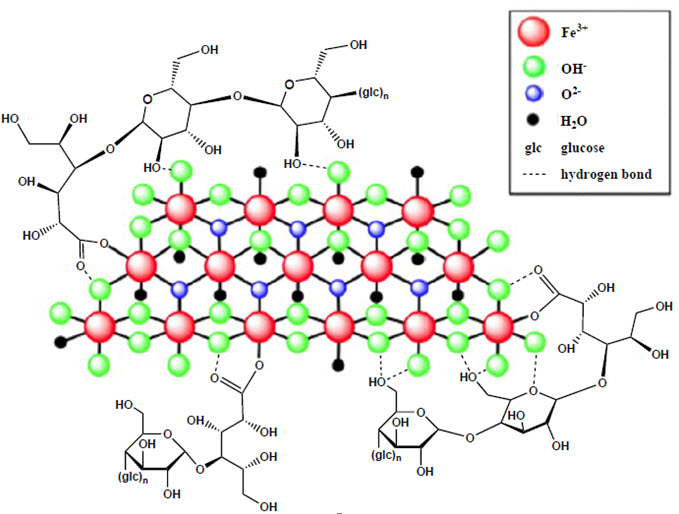
Injectafer (ferric carboxymaltose injection) is a dark brown, sterile, aqueous, isotonic colloidal solution for intravenous injection. Each mL contains 50 mg iron as ferric carboxymaltose in water for injection. Injectafer is available in 2 mL, 15 mL and 20 mL single-dose vials. Sodium hydroxide and/or hydrochloric acid may have been added to adjust the pH to 5.0-7.0.
Vial closure is not made with natural rubber latex.
Mechanism of Action
Ferric carboxymaltose is a colloidal iron (III) hydroxide in complex with carboxymaltose, a carbohydrate polymer that releases iron.
Who should not use Injectafer?
Do not receive Injectafer if you are allergic to ferric carboxymaltose or any of the ingredients in Injectafer. See the end of this leaflet for a complete list of ingredients in Injectafer.
What should I tell my healthcare provider before using Injectafer?
Before receiving Injectafer, tell your healthcare provider about all of your medical conditions, including if you:
- have had an allergic reaction to iron given into your vein
- have high blood pressure
- are pregnant or plan to become pregnant. It is not known if Injectafer will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Injectafer passes into your breast milk. It is unknown whether Injectafer would pose a risk to your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with Injectafer.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Injectafer?
Injectafer is given intravenously (into your vein) by your healthcare provider in 2 doses at least 7 days apart or given as a single dose.
What are the possible side effects of Injectafer?
Injectafer may cause serious side effects, including:
- Allergic (hypersensitivity) reactions. Serious life-threatening allergic reactions have happened in people who receive Injectafer. Other serious reactions including itching, hives, wheezing, and low blood pressure also have happened during treatment with Injectafer. Tell your healthcare provider if you have ever had any unusual or allergic reaction to any iron given by vein.
- High blood pressure (hypertension). High blood pressure, sometimes with face flushing, dizziness, or nausea, has happened during treatment with Injectafer. Your healthcare provider will check your blood pressure and check for any signs and symptoms of high blood pressure after you receive Injectafer.
The most common side effects of Injectafer include:
- nausea
- high blood pressure
- flushing
- low levels of phosphorous in your blood
- dizziness
These are not all the possible side effects of Injectafer.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Injectafer
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about Injectafer that is written for health professionals.
What are the ingredients in Injectafer?
Active ingredient: ferric carboxymaltose
Inactive ingredients: water for injection. Sodium hydroxide and/or hydrochloric acid may have been added to adjust pH to 5.0-7.0.
Label
PRINCIPAL DISPLAY PANEL – 2 ML CARTON LABELING
- NDC 0517-0602-01Injectafer® (ferric carboxymaltose injection)
100 mg/2 mL (50 mg/mL)
For Intravenous Use Only
Single Dose Vial.
Discard Unused Portion.
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
PRINCIPAL DISPLAY PANEL – 15 ML CONTAINER LABEL
- NDC 0517-0650-01Injectafer® (ferric carboxymaltose injection)
750 mg/15 mL(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Single Dose Vial. Discard Unused Portion.
Rx Only
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
SRC: NLM .