Imcivree
Generic name: setmelanotide
Dosage form: injection, for subcutaneous use
Drug class: Melanocortin receptor agonists
Medically reviewed by A Ras MD.
What is Imcivree?
Imcivree is a prescription medicine used in adults and children 6 years of age and older with obesity due to the genetic conditions proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency, to help them lose weight and keep the weight off.
Your healthcare provider should order a genetic test to confirm POMC, PCSK1, or LEPR deficiency before you start using Imcivree.
Imcivree is not for use in people with the following conditions because it may not work:
Obesity due to suspected POMC, PCSK1, or LEPR deficiency not confirmed by genetic testing (benign or likely benign result).
Other types of obesity not related to POMC, PCSK1, or LEPR deficiency, including obesity associated with other genetic conditions and general obesity.
It is not known if Imcivree is safe and effective in children under 6 years of age.
Description
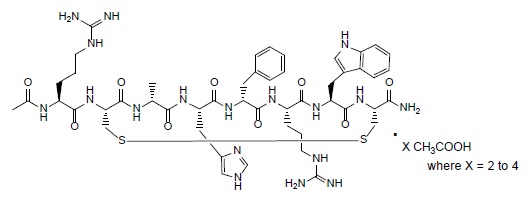
IMCIVREE contains setmelanotide acetate, a melanocortin 4 (MC4) receptor agonist. Setmelanotide is an 8 amino acid cyclic peptide analog of endogenous melanocortin peptide α-MSH (alpha-melanocyte stimulating hormone).
The chemical name for setmelanotide acetate is acetyl-L-arginyl-L-cysteinyl-D-alanyl-L-histidinyl-D-phenylalanyl-L-arginyl-L-tryptophanyl-L-cysteinamide cyclic (2→8)-disulfide acetate. Its molecular formula is C49H68N18O9S2 (anhydrous, free-base), and molecular mass is 1117.3 Daltons (anhydrous, free-base).
The chemical structure of setmelanotide acetate is:
IMCIVREE (setmelanotide) injection is a sterile clear to slightly opalescent, colorless to slightly yellow solution for subcutaneous use. Each 1 mL of IMCIVREE contains 10 mg of setmelanotide provided as setmelanotide acetate, which is a salt with 2 to 4 molar equivalents of acetate, and the following inactive ingredients: 10 mg benzyl alcohol, 8 mg carboxymethylcellulose sodium (average MWt 90,500), 1 mg edetate disodium dihydrate, 100 mg N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl- glycero-3- phosphoethanolamine sodium salt, 11 mg mannitol, 5 mg phenol, and Water for Injection. The pH of IMCIVREE is 5 to 6.
Mechanism of Action
Setmelanotide is an MC4 receptor agonist with 20-fold less activity at the melanocortin 3 (MC3) and melanocortin 1 (MC1) receptors. MC4 receptors in the brain are involved in regulation of hunger, satiety, and energy expenditure. Based on nonclinical evidence, setmelanotide may re-establish MC4 receptor pathway activity to reduce food intake and promote weight loss through decreased caloric intake and increased energy expenditure in patients with obesity due to POMC, PCSK1, or LEPR deficiency, or BBS associated with insufficient activation of the MC4 receptor. The MC1 receptor is expressed on melanocytes, and activation of this receptor leads to accumulation of melanin and increased skin pigmentation independently of ultraviolet light
What should I tell my healthcare provider before using Imcivree?
Before using Imcivree, tell your healthcare provider about all your medical conditions, including if you:
- have or have had areas of darkened skin, including skin discoloration (hyperpigmentation).
- have or have had depression, or suicidal thoughts or behavior.
- have kidney problems.
- are pregnant or planning to become pregnant. Losing weight while pregnant may harm your unborn baby. Your healthcare provider may stop your treatment with Imcivree if you become pregnant. Tell your healthcare provider if you become pregnant or think you might be pregnant during treatment with Imcivree.
- are breastfeeding or plan to breastfeed. It is not known if Imcivree passes into your breastmilk. You should not breastfeed during treatment with Imcivree.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Imcivree?
- See the detailed instructions for use to learn how to prepare and inject Imcivree.
- Imcivree is given as an injection under your skin (subcutaneous) by you or a caregiver.
- A healthcare provider should show you or your caregiver how to prepare and inject your dose of Imcivree before injecting for the first time. Do not try to inject Imcivree unless you have been trained by a healthcare provider.
- Use Imcivree exactly as prescribed by your healthcare provider.
- Your healthcare provider may tell you to stop using Imcivree if you have not lost a certain amount of weight after 12 to 16 weeks of treatment.
- Imcivree should be injected 1 time each day when you first wake up. Imcivree can be given with or without food.
- If you miss a dose of Imcivree, inject your next dose at the regularly scheduled time the next day.
What are the possible side effects of Imcivree?
Imcivree may cause serious side effects, including:
- Male and female sexual function problems. Imcivree can cause an erection that happens without any sexual activity in males (spontaneous penile erection) and unwanted sexual reactions (changes in sexual arousal that happen without any sexual activity) in females. If you have an erection lasting longer than 4 hours, get emergency medical help right away.
- Depression and suicidal thoughts or actions. You or a caregiver should call your healthcare provider right away if you have any new or worsening symptoms of depression.
- Increased skin pigmentation and darkening of skin lesions (moles or nevi) you already have. These changes happen because of how Imcivree works in the body and will go away when you stop using Imcivree. You should have a full body skin exam before starting and during treatment with Imcivree to check for skin changes.
- Benzyl alcohol toxicity. Benzyl alcohol is a preservative in Imcivree. Benzyl alcohol can cause serious side effects, including death, in premature and low-birth weight infants, who have received medicines that contain benzyl alcohol. Imcivree should not be used in premature and low-birth weight infants.
The most common side effects of Imcivree include:
- injection site reactions
- darkening of the skin
- nausea
- headache
- diarrhea
- abdominal pain
- back pain
- fatigue
- vomiting
- depression
- upper respiratory tract infection
- erection that happens without any sexual activity in males
These are not all the possible side effects of Imcivree.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or Rhythm Pharmaceuticals at 1-833-789-6337.
General information about the safe and effective use of Imcivree
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Imcivree for a condition for which it was not prescribed. Do not give Imcivree to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Imcivree that is written for health professionals.
What are the ingredients in Imcivree?
Active ingredient: setmelanotide
Inactive ingredients: N-(carbonyl-methoxypolyethylene glycol 2000)-1, 2-distearoyl- glycero-3-phosphoethanolamine sodium salt, carboxymethylcellulose sodium, mannitol, phenol, benzyl alcohol, edetate disodium dihydrate, and water for injection.
Instructions for use for Imcivree
Imcivree [im-SIV-ree]
(setmelanotide)
injection, for subcutaneous use
Important information you need to know before injecting Imcivree
- Imcivree is for injection under the skin only (subcutaneous injection). Do not inject Imcivree into a vein or muscle.
- Inject Imcivree 1 time each day when you first wake up.
- Take Imcivree with or without food.
- Imcivree is given by you or a caregiver. A healthcare provider will show you or your caregiver how to inject your dose of Imcivree before you inject for the first time. Ask your healthcare provider or call Imcivree Guidance, Partnership, Support (GPS) at 1-844-YOUR-GPS (1-844-968-7477) if you have questions.
- Store opened vials of Imcivree in the refrigerator between 36°F to 46°F (2°C to 8°C). If needed, vials may be removed from the refrigerator and stored at temperatures ranging from refrigerated to room temperature (36°F to 77°F (2°C to 25°C)), and vials may be returned to the refrigerator. Brief temperature excursions up to 86°F (30°C) are permitted. Throw away all opened vials 30 days after first opening, even if some medicine is still left.
- Unopened vials of Imcivree may be stored in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date. If needed, unopened vials may also be removed from the refrigerator and stored at temperatures ranging from refrigerated to room temperature (36°F to 77°F (2°C to 25°C)) for up to 30 days. Vials may be returned to the refrigerator. Brief storage temperature excursions up to 86°F (30°C) are permitted. Throw away Imcivree if it has been more than 30 days since the vial was first removed from the refrigerator.
- If necessary, vials may be stored at room temperature below 86°F (30° C) and may be returned to refrigerated conditions. Write the date on the carton when you first open the vial.
- Throw away Imcivree that has been stored above 86°F (30° C).
Important note:
- Only use the syringes and needles provided to you for use with Imcivree.
- Always use a new syringe and needle for each injection to prevent contamination.
- Throw away used syringes and needles in a puncture-resistant, disposable sharps container as soon as you finish giving the injection. See “Disposing of Imcivree” at the end of these instructions.
- Do not reuse or share your needles with other people.
- Do not recap the needle. Recapping the needle can lead to a needle stick injury.
- Keep Imcivree, needles, syringes, and all medicines out of the reach of children.
Understanding Your Imcivree Dose
Calculate the number of doses of Imcivree in each vial:
- Each unopened Imcivree vial contains 10 milligrams (mg) of medicine in 1 milliliter (mL) of solution.
- The vial will contain both medicine and air. Most of the vial will be filled with air.
- Your healthcare provider will determine your dose of medicine in milligrams (mg).
- The Imcivree vial may be used to give more than 1 dose of medicine (multiple-dose vial).
- Use Figure A to see how many times you may use each vial based on your prescribed dose.
- Do not use more doses from a single vial than listed in Figure A

Preparing to Inject Imcivree
Step 1 Gather your supplies.
- Gather the supplies you will need for your injection (Figure B).
- Place your supplies on a clean, flat work surface.

| Step 2 Check your Imcivree vial. | |
 |
|
| Step 3 Prepare your Imcivree vial. | |
 |
|
 |
|
 |
|
| Step 4 Prepare the syringe.
When measuring your dose, be sure to read the markings starting from the end closest to the black rubber stopper (Figure G).
|
|
 |
|
 |
|
 |
|
 |
|
| What to do if you see large air bubbles:
Large air bubbles can reduce the dose of medicine you receive. To remove large air bubbles:
|
|
 |
|
Injecting ImcivreeStep 5 Prepare your injection site. |
|

|
|
|
|
| Rotate your injection site each day.
You should use a different injection site each time you give an injection, at least 1 inch away from the area you used for your previous injection. You may want to use a calendar or diary to record your injection sites. |
|
| Step 6 Place your hands for the injection. | |
 |
|
| Step 7 Inject and release. | |
 |
|
 |
|
| Tips for giving injections to children
When giving a child an injection, it can help to have the child do other things. Have the child:
|
|
Storing Imcivree
|
|
Disposing of Imcivree |
|
 |
|
Label
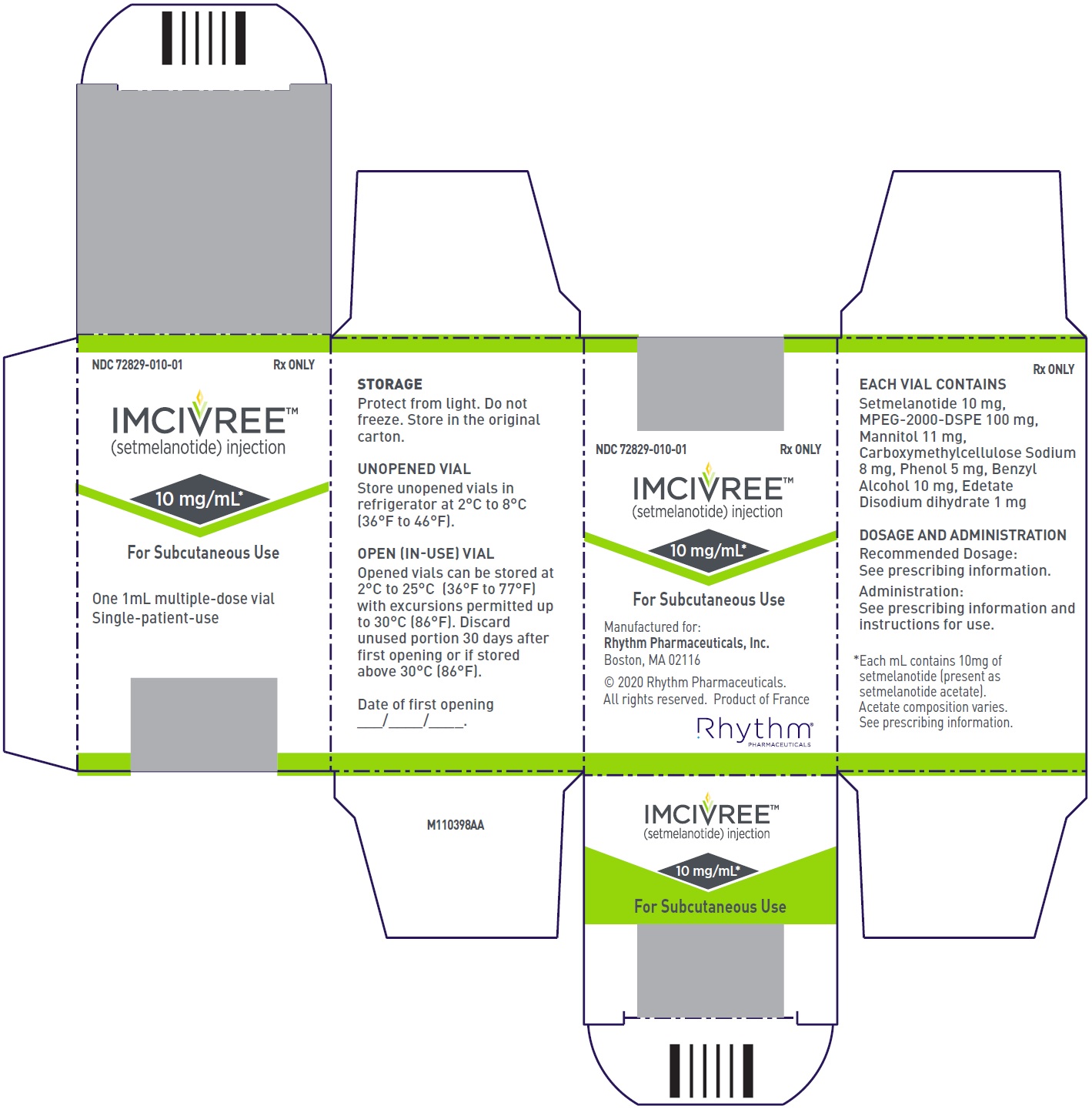
PRINCIPAL DISPLAY PANEL – NDC: 72829-010-01 – CARTON LABEL
PRINCIPAL DISPLAY PANEL – NDC: 72829-010-01 – VIAL LABEL