Zontivity
Generic name: vorapaxar
Drug class: Protease-activated receptor-1 antagonists
Medically reviewed by A Ras MD.
What is Zontivity?
Zontivity is a prescription medicine used to treat people who have had a heart attack or reduced blood flow in their legs (peripheral arterial disease).
Zontivity is used with aspirin and/or clopidogrel to lower your chance of having another serious problem with your heart or blood vessels, such as heart attack, stroke, or death.
It is not known if Zontivity is safe and effective in children.
Description
ZONTIVITY contains vorapaxar sulfate, a tricyclic himbacine-derived selective inhibitor of platelet aggregation mediated by PAR-1.
The chemical name of vorapaxar sulfate is ethyl [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-{(1E)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethen-1-yl}-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl]carbamate sulfate. The empirical formula is C 29H 33FN 2O 4∙H 2SO 4, and its molecular weight is 590.7. The structural formula is:
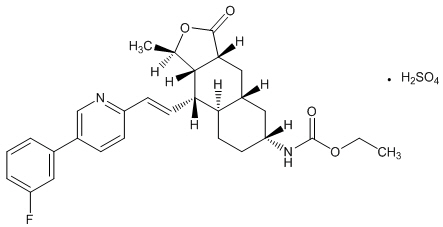
Vorapaxar sulfate is a white to off-white solid. Vorapaxar sulfate is freely soluble in methanol and slightly soluble in ethanol, acetone, 2-propanol, and acetonitrile. In aqueous solution, it is slightly soluble in pH 1; its solubility decreases with increasing pH. ZONTIVITY tablets are formulated with vorapaxar sulfate, but during manufacture and storage, partial conversion from vorapaxar sulfate to vorapaxar free base may occur.
ZONTIVITY is available for oral use as tablets containing 2.08 mg of vorapaxar, which is equivalent to 2.5 mg of vorapaxar sulfate.
Each film-coated tablet of ZONTIVITY contains the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, and magnesium stearate. In addition, the film coating contains the following inactive ingredients: lactose monohydrate, hypromellose, titanium dioxide, triacetin (glycerol triacetate), and iron oxide yellow.
Mechanism of Action
Vorapaxar is a reversible antagonist of the protease-activated receptor-1 (PAR-1) expressed on platelets, but its long half-life makes it effectively irreversible. Vorapaxar inhibits thrombin-induced and thrombin receptor agonist peptide (TRAP)-induced platelet aggregation in in vitro studies. Vorapaxar does not inhibit platelet aggregation induced by adenosine diphosphate (ADP), collagen or a thromboxane mimetic and does not affect coagulation parameters ex vivo. PAR-1 receptors are also expressed in a wide variety of cell types, including endothelial cells, neurons, and smooth muscle cells, but the pharmacodynamic effects of vorapaxar in these cell types have not been assessed.
What is the most important information I should know about Zontivity?
Zontivity is used to lower your chance of having another serious problem with your heart or blood vessels, but Zontivity (and similar drugs) can cause bleeding that can be serious and lead to death.
Call your doctor right away if you have any of these signs or symptoms of bleeding while taking Zontivity:
- bleeding that is severe or that you cannot control
- pink, red, or brown urine
- vomiting blood or your vomit looks like “coffee grounds”
- red or black stools (looks like tar)
- coughing up blood or blood clots.
While you take Zontivity and for about 4 weeks after your treatment with Zontivity is stopped:
- you may bruise and bleed more easily (nose bleeds may be common)
- it will take longer than usual for any bleeding to stop.
Who should not take Zontivity?
Do not take Zontivity if you:
- have had a stroke or “mini stroke” (also known as transient ischemic attack or TIA)
- have had bleeding in your brain
- currently have unusual bleeding, such as bleeding in your head, stomach or intestines (an ulcer).
If you have a stroke, TIA, or bleeding in your brain while taking Zontivity your doctor should stop your treatment with Zontivity. Follow your doctor’s instructions about stopping Zontivity.
Do not stop taking Zontivity without talking to the doctor who prescribed it for you.
What should I tell my healthcare provider before taking Zontivity?
Before you take Zontivity, tell your doctor if you:
- have had bleeding problems or history of stomach ulcers
- have had a stroke or “mini-stroke” (also known as transient ischemic attack or TIA)
- have had any recent serious injury or surgery
- plan to have surgery or a dental procedure
- have kidney problems or severe liver problems
- are pregnant or plan to become pregnant. It is not known if Zontivity will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Zontivity passes into your breast milk. You and your doctor should decide if you will take Zontivity or breastfeed. You should not do both.
Tell all of your doctors and dentists that you are taking Zontivity. They should talk to the doctor who prescribed Zontivity for you before you have any surgery or dental procedure.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, dietary or herbal supplements. Taking Zontivity with certain other medicines may increase your risk of bleeding and may affect how Zontivity works.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Zontivity?
Take Zontivity exactly as prescribed by your doctor.
- Take Zontivity 1 time each day.
- Take Zontivity with or without food.
- Take Zontivity with aspirin and/or clopidogrel as prescribed by your doctor.
- Do not stop taking Zontivity without first talking to the doctor who prescribed it for you.
- If you take too much Zontivity, call your doctor, or go to the nearest emergency room right away.
What are the possible side effects of Zontivity?
- See “What is the most important information I should know about Zontivity?”
- Anemia (low level of red blood cells)
- Depression
- Rash
These are not all the possible side effects of Zontivity.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Zontivity?
- Store Zontivity at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Zontivity in the bottle it comes in.
- Keep the bottle tightly closed.
- The Zontivity bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Keep the desiccant packet in the bottle. Do not throw away the desiccant packet.
- Store blister packs of Zontivity in the original package it comes in.
Keep Zontivity and all medicines out of the reach of children.
You can ask your doctor or pharmacist for information about Zontivity that is written for health professionals.
For more information, go to www.zontivity.com or call 1-833-694-8235.
What are the ingredients in Zontivity?
Active ingredient: vorapaxar sulfate
Inactive ingredients:
Tablet: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, and magnesium stearate
Film coating: lactose monohydrate, hypromellose, titanium dioxide, triacetin (glycerol triacetate), and iron oxide yellow.
Label
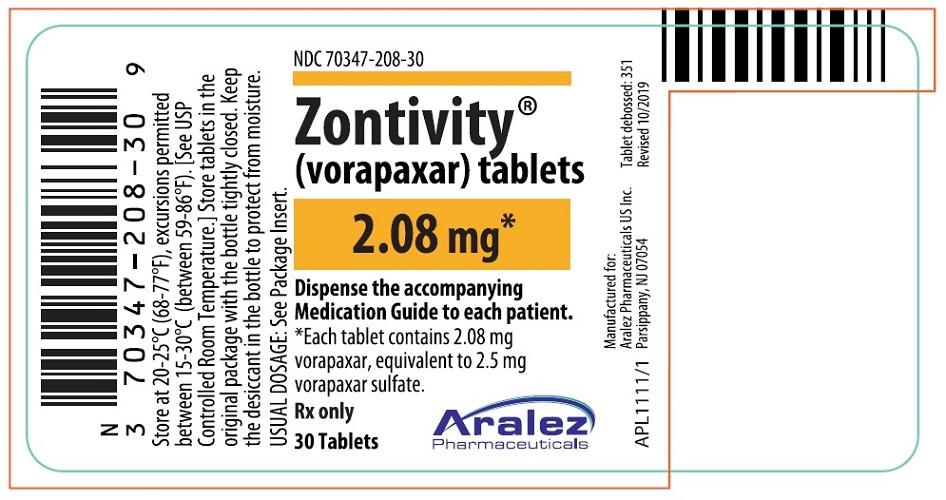
PRINCIPAL DISPLAY PANEL – 30 Tablet Bottle Label
- NDC 70347-208-30
- Zontivity ®
(vorapaxar) tablets - 2.08 mg*
- Dispense the accompanying
Medication Guide to each patient. - *Each tablet contains 2.08 mg
vorapaxar, equivalent to 2.5 mg
vorapaxar sulfate. - Rx only
- 30 Tablets
- Aralez Pharmaceuticals

SRC: NLM .