Venclexta
Generic name: venetoclax
Brand names: Venclexta, Venclexta Starting Pack
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Venclexta?
Venclexta is a prescription medicine used to treat adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
It is also used in combination with azacitidine, or decitabine, or low-dose cytarabine to treat adults with newly-diagnosed acute myeloid leukemia (AML) who are 75 years of age or older or have other medical conditions that prevent the use of standard chemotherapy.
It is not known if Venclexta is safe and effective in children.
Description
Venetoclax is a BCL-2 inhibitor. It is a light yellow to dark yellow solid with the empirical formula C45H50ClN7O7S and a molecular weight of 868.44. Venetoclax is described chemically as 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide) and has the following chemical structure:
![the following chemical structure for Venetoclax is a BCL-2 inhibitor. It is a light yellow to dark yellow solid with the empirical formula C45H50ClN7O7S and a molecular weight of 868.44. Venetoclax is described chemically as 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide).](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=venetoclax-spl-01.jpg&setid=b118a40d-6b56-cee3-10f6-ded821a97018)
Venetoclax has very low aqueous solubility.
VENCLEXTA tablets for oral use are supplied as pale yellow or beige tablets that contain 10, 50, or 100 mg venetoclax as the active ingredient. Each tablet also contains the following inactive ingredients: copovidone, colloidal silicon dioxide, polysorbate 80, sodium stearyl fumarate, and calcium phosphate dibasic. In addition, the 10 mg and 100 mg coated tablets include the following: iron oxide yellow, polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide.
The 50 mg coated tablets also include the following: iron oxide yellow, iron oxide red, iron oxide black, polyvinyl alcohol, talc, polyethylene glycol and titanium dioxide. Each tablet is debossed with “V” on one side and “10”, “50” or “100” corresponding to the tablet strength on the other side.
Mechanism of Action
Venetoclax is a selective and orally bioavailable small-molecule inhibitor of BCL-2, an anti-apoptotic protein. Overexpression of BCL-2 has been demonstrated in CLL and AML cells where it mediates tumor cell survival and has been associated with resistance to chemotherapeutics. Venetoclax helps restore the process of apoptosis by binding directly to the BCL-2 protein, displacing pro-apoptotic proteins like BIM, triggering mitochondrial outer membrane permeabilization and the activation of caspases. In nonclinical studies, venetoclax has demonstrated cytotoxic activity in tumor cells that overexpress BCL-2.
What is the most important information I should know about Venclexta?
Venclexta can cause serious side effects, including:
Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure, the need for dialysis treatment, and may lead to death. Your healthcare provider will do tests to check your risk of getting TLS before you start taking Venclexta. You will receive other medicines before starting and during treatment with Venclexta to help reduce your risk of TLS. You may also need to receive intravenous (IV) fluids into your vein. Your healthcare provider will do blood tests to check for TLS when you first start treatment and during treatment with Venclexta. It is important to keep your appointments for blood tests. Tell your healthcare provider right away if you have any symptoms of TLS during treatment with Venclexta, including:
- fever
- chills
- nausea
- vomiting
- confusion
- shortness of breath
- seizures
- irregular heartbeat
- dark or cloudy urine
- unusual tiredness
- muscle or joint pain
Drink plenty of water during treatment with Venclexta to help reduce your risk of getting TLS.
Drink 6 to 8 glasses (about 56 ounces total) of water each day, starting 2 days before your first dose, on the day of your first dose of Venclexta, and each time your dose is increased.
Your healthcare provider may delay, decrease your dose, or stop treatment with Venclexta if you have side effects.
See “What are the possible side effects of Venclexta?” for more information about side effects.
Who should not take Venclexta?
Certain medicines must not be taken when you first start taking Venclexta and while your dose is being slowly increased because of the risk of increased tumor lysis syndrome (TLS).
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Venclexta and other medicines may affect each other causing serious side effects.
- Do not start new medicines during treatment with Venclexta without first talking with your healthcare provider.
What should I tell my healthcare provider before taking Venclexta?
Before taking Venclexta, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems
- have liver problems
- have problems with your body salts or electrolytes, such as potassium, phosphorus, or calcium
- have a history of high uric acid levels in your blood or gout
- are scheduled to receive a vaccine. You should not receive a “live vaccine” before, during, or after treatment with Venclexta, until your healthcare provider tells you it is okay. If you are not sure about the type of immunization or vaccine, ask your healthcare provider. These vaccines may not be safe or may not work as well during treatment with Venclexta.
- are pregnant or plan to become pregnant. Venclexta may harm your unborn baby.
- If you are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Venclexta.
- Females who are able to become pregnant should use effective birth control during treatment and for at least 30 days after the last dose of Venclexta.
- If you become pregnant or think you are pregnant, tell your healthcare provider right away.
- are breastfeeding or plan to breastfeed. It is not known if Venclexta passes into your breast milk. Do not breastfeed during treatment with Venclexta.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Venclexta and other medicines may affect each other causing serious side effects. See “Who should not take Venclexta?”
How should I take Venclexta?
- Take Venclexta exactly as your healthcare provider tells you to take it. Do not change your dose of Venclexta or stop taking Venclexta unless your healthcare provider tells you to.
- When you first take Venclexta:
- You may need to take Venclexta at a hospital or clinic to be monitored for TLS.
- If you are taking Venclexta for CLL or SLL, your healthcare provider will start Venclexta at a low-dose. Your dose will be slowly increased weekly over 5 weeks up to the full dose. Read the Quick Start Guide that comes with Venclexta before your first dose.
- If you are taking Venclexta for AML, your healthcare provider will start Venclexta at a low-dose. Your dose will be slowly increased daily up to the full dose. Follow your healthcare provider’s instructions carefully while increasing to the full dose.
- Follow the instructions about drinking water described in the section of this Medication Guide about TLS called “What is the most important information I should know about Venclexta?” and also in the Quick Start Guide.
- Take Venclexta 1 time a day with a meal and water at about the same time each day.
- Swallow Venclexta tablets whole. Do not chew, crush, or break the tablets.
- If you miss a dose of Venclexta and it has been less than 8 hours, take your dose as soon as possible. If you miss a dose of Venclexta and it has been more than 8 hours, skip the missed dose and take the next dose at your usual time.
- If you vomit after taking Venclexta, do not take an extra dose. Take the next dose at your usual time the next day.
What should I avoid while taking Venclexta?
You should not drink grapefruit juice, eat grapefruit, Seville oranges (often used in marmalades), or starfruit while you are taking Venclexta. These products may increase the amount of Venclexta in your blood.
What are the possible side effects of Venclexta?
Venclexta can cause serious side effects, including:
- See “What is the most important information I should know about Venclexta?”
- Low white blood cell count (neutropenia). Low white blood cell counts are common with Venclexta but can also be severe. Your healthcare provider will do blood tests to check your blood counts during treatment with Venclexta.
- Infections. Death and serious infections such as pneumonia and blood infection (sepsis) have happened during treatment with Venclexta. Your healthcare provider will closely monitor and treat you right away if you have fever or any signs of infection during treatment with Venclexta.
Tell your healthcare provider right away if you have a fever or any signs of an infection during treatment with Venclexta.
The most common side effects of Venclexta when used in combination with obinutuzumab or rituximab or alone in people with CLL or SLL include:
- low platelet counts
- low red blood cell counts
- diarrhea
- nausea
- upper respiratory tract infection
- cough
- muscle and joint pain
- tiredness
- swelling of your arms, legs, hands, and feet
The most common side effects of Venclexta in combination with azacitidine or decitabine or low-dose cytarabine in people with AML include:
- nausea
- diarrhea
- low platelet counts
- constipation
- fever with low white blood cell counts
- low red blood cell counts
- infection in blood
- rash
- dizziness
- low blood pressure
- fever
- swelling of your arms, legs, hands, and feet
- vomiting
- tiredness
- shortness of breath
- bleeding
- infection in lung
- stomach (abdominal) pain
- pain in muscles or back
- cough
- sore throat
Venclexta may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of Venclexta. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Venclexta
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Venclexta for a condition for which it was not prescribed. Do not give Venclexta to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Venclexta that is written for health professionals.
How should I store Venclexta?
- Store Venclexta at or below 86°F (30°C).
- For people with CLL/SLL, keep Venclexta tablets in the original package during the first 4 weeks of treatment. Do not transfer the tablets to a different container.
Keep Venclexta and all medicines out of reach of children.
What are the ingredients in Venclexta?
Active ingredient: venetoclax
Inactive ingredients: copovidone, colloidal silicon dioxide, polysorbate 80, sodium stearyl fumarate, and calcium phosphate dibasic.
The 10 mg and 100 mg coated tablets also include: iron oxide yellow, polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide.
The 50 mg coated tablets also include: iron oxide yellow, iron oxide red, iron oxide black, polyvinyl alcohol, talc, polyethylene glycol, and titanium dioxide.
Label
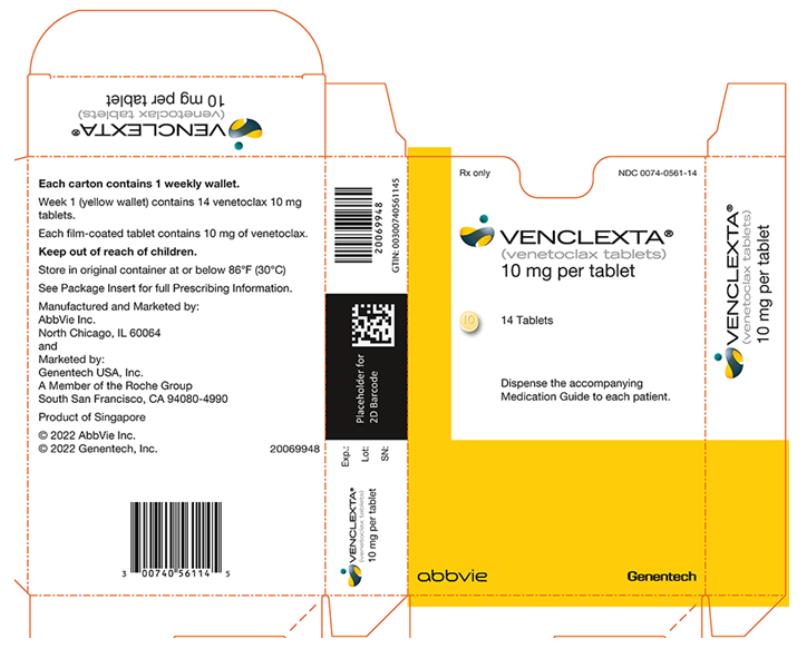
PRINCIPAL DISPLAY PANEL
- NDC 0074-0561-14
- Rx only
- VENCLEXTA®
- (venetoclax tablets)
- 10 mg per tablet
- 14 Tablets
- Dispense the accompanying Medication Guide to each patient.
- abbvie
- Genentech
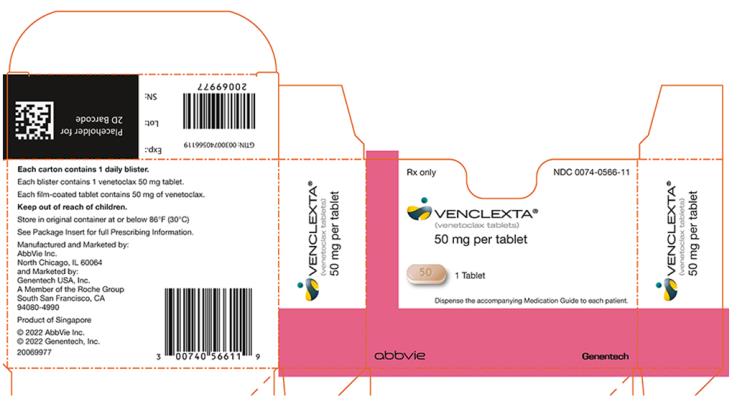
PRINCIPAL DISPLAY PANEL
- NDC 0074-0566-11
- Rx only
- VENCLEXTA®
- (venetoclax tablets)
- 50 mg per tablet
- 1 Tablet
- Dispense the accompanying Medication Guide to each patient.
- abbvie
- Genentech
PRINCIPAL DISPLAY PANEL
- NDC 0074-0576-22
- Rx only
- VENCLEXTA®
- (venetoclax tablets)
- 100 mg
- Attention: Dispense and store Venclexta in original container to protect from moisture.
- 120 Tablets
- Dispense the accompanying Medication Guide to each patient.
- abbvie
- Genetech

SRC: NLM .