Jemperli
Generic name: dostarlimab-gxly
Dosage form: injection
Drug class: Anti-PD-1 monoclonal antibodies
Medically reviewed by A Ras MD.
What is Jemperli?
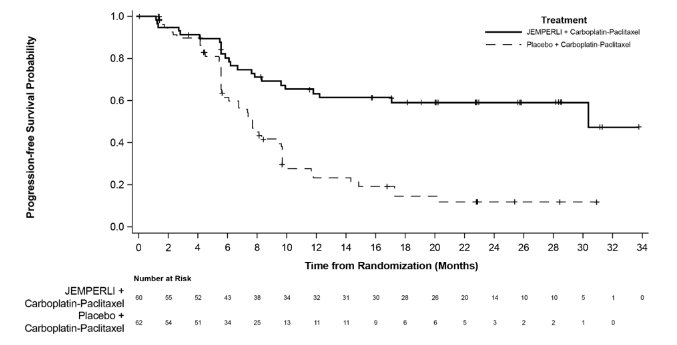
Jemperli is a prescription medicine used to treat adults with certain cancers that have been shown by a laboratory test to be mismatch repair deficient (dMMR), and your cancer has returned, or it has spread or cannot be removed by surgery (advanced cancer).
Jemperli may be used when you have a kind of uterine cancer called endometrial cancer, and you have received chemotherapy that contains platinum and it did not work or is no longer working, you have a solid tumor that progressed during treatment or after treatment, and you have no satisfactory treatment options.
It is not known if Jemperli is safe and effective in children.
Description
Dostarlimab-gxly is a programmed death receptor-1 (PD-1)–blocking IgG4 humanized monoclonal antibody. Dostarlimab‑gxly is produced in Chinese hamster ovary cells and has a calculated molecular weight of about 144 kDa.
JEMPERLI (dostarlimab-gxly) injection is a sterile, clear to slightly opalescent, colorless to yellow solution essentially free from visible particles. It is supplied as single-dose vials.
Each vial contains 500 mg of JEMPERLI in 10 mL of solution with a pH of 6. Each mL of solution contains 50 mg of dostarlimab-gxly, citric acid monohydrate (0.48 mg), L-arginine hydrochloride (21.07 mg), polysorbate 80 (0.2 mg), sodium chloride (1.81 mg), trisodium citrate dihydrate (6.68 mg), and Water for Injection, USP.
Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors, and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Dostarlimab-gxly is a humanized monoclonal antibody of the IgG4 isotype that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
What is the most important information I should know about Jemperli?
Jemperli is a medicine that may treat certain cancers by working with your immune system. Jemperli can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended.
Call or see your healthcare provider right away if you develop any new or worsening signs or symptoms, including:
- Lung problems.
- cough
- shortness of breath
- chest pain
- Intestinal problems.
- diarrhea or more bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
- Liver problems.
- yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than usual
- Hormone gland problems.
- headaches that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
- Kidney problems.
- change in the amount or color of your urine
- blood in your urine
- swelling in your ankles
- loss of appetite
- Skin problems.
- rash
- itching
- skin blistering or peeling
- painful sores or ulcers in your mouth or in your nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
- Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with Jemperli. Call or see your healthcare provider right away for any new or worse signs or symptoms.
- chest pain, irregular heartbeat, shortness of breath, swelling of ankles
- confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- double vision, blurry vision, sensitivity to light, eye pain, changes in eyesight
- persistent or severe muscle pain or weakness, muscle cramps
- low red blood cells, bruising
- Infusion reactions that can sometimes be severe or life-threatening. Signs and symptoms of infusion reactions may include:
- chills or shaking
- itching or rash
- flushing
- shortness of breath or wheezing
- dizziness
- feel like passing out
- fever
- back or neck pain
- Rejection of a transplanted organ. Your healthcare provider should tell you what signs and symptoms you should report and monitor you, depending on the type of organ transplant that you have had.Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with Jemperli. Your healthcare provider will monitor you for these complications.
Getting medical treatment right away may help keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during treatment with Jemperli. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with Jemperli, if you have severe side effects.
What should I tell my healthcare provider before using Jemperli?
Before you receive Jemperli, tell your healthcare provider if you have any medical conditions, including if you:
- have immune system problems, such as Crohn’s disease, ulcerative colitis, or lupus.
- have received an organ transplant.
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic).
- have received radiation treatment to your chest area.
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome.
- are pregnant or plan to become pregnant. Jemperli can harm your unborn baby.Females who are able to become pregnant:
- Your healthcare provider will do a pregnancy test before you start treatment with Jemperli.
- You should use an effective method of birth control during your treatment and for 4 months after your last dose of Jemperli. Talk to your healthcare provider about birth control methods that you can use during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Jemperli.
- are breastfeeding or plan to breastfeed. It is not known if Jemperli passes into your breast milk. Do not breastfeed during treatment and for 4 months after your last dose of Jemperli.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Jemperli?
- Your healthcare provider will give you Jemperli into your vein through an intravenous (IV) line over 30 minutes.
- Jemperli is usually given every 3 weeks for the first 4 doses, and then beginning 3 weeks later, it is usually given every 6 weeks.
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider will do blood tests to check you for side effects.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of Jemperli?
Jemperli can cause serious side effects.
- See “What is the most important information I should know about Jemperli?”
The most common side effects of Jemperli in people with dMMR solid tumors include:
- tiredness and weakness
- low red blood cell count (anemia)
- diarrhea
- nausea
- decreased number of certain white blood cells
- decreased albumin in the blood
- increase in certain liver blood tests
- decreased salt (sodium) in the blood
These are not all the possible side effects of Jemperli. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Jemperli
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about Jemperli, talk with your healthcare provider. You can ask your healthcare provider for information about Jemperli that is written for healthcare professionals.
What are the ingredients in Jemperli?
Active ingredient: dostarlimab-gxly
Inactive ingredients: citric acid monohydrate, L-arginine hydrochloride, polysorbate 80, sodium chloride, trisodium citrate dihydrate, and Water for Injection.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0898-03
- Jemperli
- (dostarlimab-gxly) Injection
- 500 mg/10 mL
- (50 mg/mL)
- gsk
- For Intravenous Infusion after Dilution
- Single-Dose Vial
- Dosage: See Prescribing Information.
- Storage: Refrigerate at 2°C to 8°C (36°F to 46°F) in original carton to protect from light.
- Do not freeze or shake.
- Discard unused portion.
- Do not accept if plastic overseal is missing or not securely fitted.
- ©2020 GSK group of companies or its licensor.
- Rev. 10/20
- PCR-700-13182