Viibryd
Generic name: vilazodone
Brand names: Viibryd, Viibryd Starter
Drug class: Miscellaneous antidepressants
Medically reviewed by A Ras MD.
What is Viibryd?
Viibryd is a prescription medicine used to treat a certain type of depression called Major Depressive Disorder (MDD) in adults. It is not known if Viibryd is safe and effective for use in children for the treatment of MDD.
Description
VIIBRYD tablets for oral administration contain polymorph Form IV vilazodone hydrochloride (HCl), a selective serotonin reuptake inhibitor and a 5HT1A receptor partial agonist.
Vilazodone HCl is 2-benzofurancarboxamide, 5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piprazinyl]-, hydrochloride (1:1). Its molecular weight is 477.99. The structural formula is:
![The structural formula for Vilazodone HCl is 2-benzofurancarboxamide, 5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-, hydrochloride (1:1). Its molecular weight is 477.99.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=viibryd-01.jpg&setid=4c55ccfb-c4cf-11df-851a-0800200c9a66)
VIIBRYD tablets are available as 10 mg, 20 mg, and 40 mg film-coated tablets containing 10 mg, 20 mg, and 40 mg of vilazodone HCl, respectively.
In addition to the active ingredient, VIIBRYD tablets contain the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, FD&C Blue #1 (40 mg only), FD&C Red #40 (10 mg only), and FD&C Yellow #6 (20 mg only).
Mechanism of action
The mechanism of action of vilazodone in the treatment of major depressive disorder is not fully understood, but is thought to be related to its enhancement of serotonergic activity in the CNS through selective inhibition of serotonin reuptake. Vilazodone is also a partial agonist at serotonergic 5-HT1A receptors; however, the net result of this action on serotonergic transmission and its role in vilazodone’s antidepressant effect are unknown.
What is the most important information I should know about Viibryd?
Viibryd may cause serious side effects, including:
- Increased risk of suicidal thoughts or actions in some children, adolescents, and young adults. Viibryd and other antidepressant medicines may increase suicidal thoughts or actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed. Viibryd is not for use in children.
- Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions. Some people may have a higher risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression, bipolar illness (also called manic-depressive illness) or have a history of suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions?
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call your healthcare provider or get emergency medical help right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
- attempts to commit suicide
- acting aggressive, being angry or violent
- new or worse depression
- panic attacks
- new or worse irritability
- an extreme increase in activity or talking (mania)
- acting on dangerous impulses
- thoughts about suicide or dying
- new or worse anxiety
- feeling agitated or restless
- trouble sleeping (insomnia)
- other unusual changes in behavior or mood
Who should not take Viibryd?
Do not take Viibryd if you:
- take a Monoamine Oxidase Inhibitor (MAOI)
- have stopped taking an MAOI in the last 14 days
- are being treated with the antibiotic linezolid or intravenous methylene blue
Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for at least 14 days after you stop treatment with Viibryd.
What should I tell my healthcare provider before taking Viibryd?
Before taking Viibryd, tell your healthcare provider about all your medical conditions, including if you:
- have or have a family history of suicide, depression, bipolar disorder, mania or hypomania
- have or had bleeding problems
- have or had seizures or convulsions
- have high pressure in the eye (glaucoma)
- have low sodium levels in your blood
- drink alcohol
- are pregnant or plan to become pregnant. Taking Viibryd late in pregnancy may lead to an increased risk of certain problems in your newborn. Talk to your healthcare provider about the risks to your baby if you take Viibryd during pregnancy. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Viibryd.
- There is a pregnancy registry for females who are exposed to Viibryd during pregnancy. The purpose of the registry is to collect information about the health of females exposed to Viibryd and their baby. If you become pregnant during treatment with Viibryd, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185.
- are breastfeeding or plan to breastfeed. It is not known if Viibryd passes into breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with Viibryd.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Viibryd and some medicines may affect each other causing possible serious side effects. Viibryd may affect the way other medicines work and other medicines may affect the way Viibryd works.
Especially tell your healthcare provider if you take:
- MAOIs
- medicines used to treat migraine headaches known as triptans
- tricyclic antidepressants
- fentanyl
- lithium
- tramadol
- tryptophan
- buspirone
- amphetamines
- St. John’s Wort
- medicines that can affect blood clotting such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) and warfarin
- diuretics
- medicines used to treat mood, anxiety, psychotic or thought disorders, including selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs)
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take Viibryd with your other medicines.
Do not start or stop any other medicines during treatment with Viibryd without talking to your healthcare provider first. Stopping Viibryd suddenly may cause you to have serious side effects. See, “What are the possible side effects of Viibryd?”
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Viibryd?
- Take Viibryd exactly as your healthcare provider tells you to. Do not change your dose or stop taking Viibryd without first talking to your healthcare provider.
- Your healthcare provider may need to change the dose of Viibryd until it is the right dose for you.
- Take Viibryd 1 time each day with food.
- If you miss a dose of Viibryd, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of Viibryd at the same time.
- If you take too much Viibryd, call your healthcare provider or poison control center at 1-800-222-1222 right away, or get emergency treatment right away.
What should I avoid while taking Viibryd?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Viibryd affects you. Viibryd can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly.
- Avoid drinking alcohol during treatment with Viibryd.
What are the possible side effects of Viibryd?
Viibryd may cause serious side effects, including:
- See “What is the most important information I should know about Viibryd?”
- Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome can happen when Viibryd is taken with certain other medicines. See, “Who should not take Viibryd?” Stop taking Viibryd and call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of serotonin syndrome:
- agitation
- confusion
- fast heartbeat
- dizziness
- flushing
- tremors, stiff muscles, or muscle twitching
- seizures
- seeing or hearing things that are not real (hallucinations)
- coma
- blood pressure changes
- sweating
- high body temperature (hyperthermia)
- loss of coordination
- nausea, vomiting, diarrhea
- Increased risk of bleeding. Taking Viibryd with aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), warfarin or blood thinners may add to this risk. Tell your healthcare provider right away about any unusual bleeding or bruising.
- Mania or hypomania (manic episodes) in people who have a history of bipolar disorder. Symptoms may include:
- greatly increased energy
- racing thoughts
- unusually grand ideas
- talking more or faster than usual
- severe trouble sleeping
- reckless behavior
- excessive happiness or irritability
- Discontinuation syndrome. Suddenly stopping Viibryd may cause you to have serious side effects. Your healthcare provider may want to decrease your dose slowly. Symptoms may include:
- nausea
- changes in your mood
- irritability and agitation
- dizziness
- electric shock sensation (paresthesia)
- anxiety
- confusion
- sweating
- headache
- tiredness
- problems sleeping
- hypomania
- ringing in your ears (tinnitus)
- seizures
- Seizures (convulsions).
- Eye problems (angle-closure glaucoma): Viibryd may cause a certain type of eye problem called angle-closure glaucoma. Call your healthcare provider if you have changes in your vision or eye pain.
- Low sodium levels in your blood (hyponatremia). Low sodium levels in your blood may be serious and may cause death. Elderly people may be at greater risk for this. Signs and Symptoms of low sodium levels in your blood may include:
- headache
- memory changes
- weakness and unsteadiness on your feet which can lead to falls
- difficulty concentrating
- confusion
In severe or more sudden cases, signs and symptoms include:
- hallucinations (seeing or hearing things that are not real)
- seizures
- respiratory arrest
- fainting
- coma
- death
The most common side effects of Viibryd include diarrhea, nausea or vomiting, trouble sleeping.
These are not all the possible side effects of Viibryd.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Viibryd
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Viibryd for a condition for which it was not prescribed. Do not give Viibryd to other people, even if they have the same symptoms that you have. It may harm them. You may ask your healthcare provider or pharmacist for information about Viibryd that is written for healthcare professionals.
How should I store Viibryd?
- Store Viibryd at room temperature (68°F to 77°F or 20°C to 25°C).
- Keep Viibryd and all medicines out of the reach of children.
What are the ingredients in Viibryd?
Active ingredient: vilazodone hydrochloride
Inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, FD&C Blue #1 (40 mg only), FD&C Red #40 (10 mg only) and FD&C Yellow #6 (20 mg only)
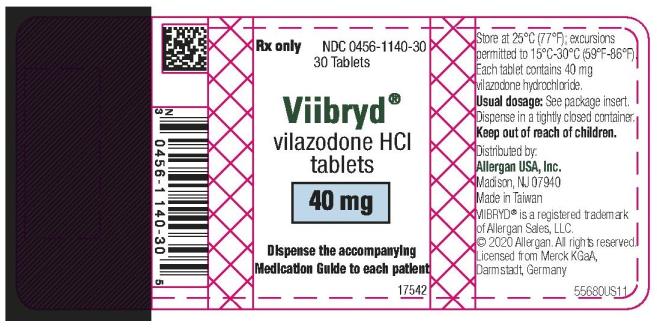
Label
PRINCIPAL DISPLAY PANEL
- NDC 0456-1110-30
Viibryd
vilazodone HCI
tablets
30 Tablets
10 mg
Rx Only
![]()
PRINCIPAL DISPLAY PANEL
- NDC 0456-1120-30
Viibryd
vilazodone HCI
tablets
30 Tablets
20 mg
Rx Only

![]()
PRINCIPAL DISPLAY PANEL
