Liletta
Generic name: levonorgestrel intrauterine system
Brand names: Kyleena, Liletta, Mirena, Skyla
Drug classes: Contraceptives, Progestins
Medically reviewed by A Ras MD.
What is Liletta?
Liletta is a hormone-releasing system placed in your uterus by your healthcare professional to prevent pregnancy for up to 6 years. Liletta can be removed by your healthcare professional at any time. Liletta can be used whether or not you have given birth to a child.
Liletta is a small, flexible plastic T-shaped system that slowly releases a progestin hormone called levonorgestrel (LNG) that is often used in birth control pills. Because Liletta releases LNG into your uterus, only small amounts of the hormone enter your blood. Liletta does not contain estrogen.
Two thin threads are attached to the stem (lower end) of Liletta. The threads are the only part of Liletta you can feel when Liletta is in your uterus; however, unlike a tampon string, the threads do not extend outside your body.

Liletta is small.

Liletta is flexible.
Description
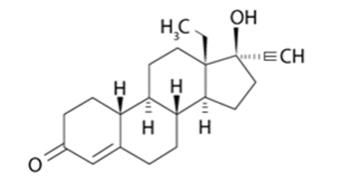
LILETTA (levonorgestrel-releasing intrauterine system) contains 52 mg of levonorgestrel, a progestin, and is intended to provide an initial release rate of 20.1 mcg/day of levonorgestrel.
Levonorgestrel USP, (-)-13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one, the active ingredient in LILETTA, is the levorotatory form of norgestrel, which consists of a racemic mixture of D-(–)-norgestrel (levonorgestrel) and L-(+)-norgestrel (dextronorgestrel). It has a molecular weight of 312.45, a molecular formula of C21H28O2, and the following structural formula:
 |
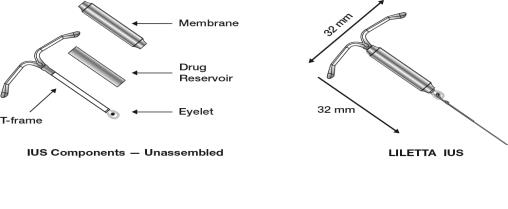
LILETTA consists of a T-shaped polyethylene frame (T-frame) with a drug reservoir around the vertical stem (Figure 15). The T-frame has a loop at one end of the vertical stem and two horizontal arms at the other end. The drug reservoir consists of a cylinder, made of a mixture of 52 mg levonorgestrel and polydimethylsiloxane (PDMS) formed from silicone base, tetra-n-propyl silicate, and stannous octoate. The drug reservoir is covered by a translucent PDMS membrane. The low-density polyethylene of the T-frame is compounded with barium sulfate, which makes it radio-opaque. A blue polypropylene monofilament removal thread is attached to an eyelet at the end of the vertical stem of the T-frame. The polypropylene of the removal thread contains a copper-containing pigment as a colorant. The components of LILETTA, including its packaging, are not manufactured using natural rubber latex.
Figure 15: Diagram of LILETTA
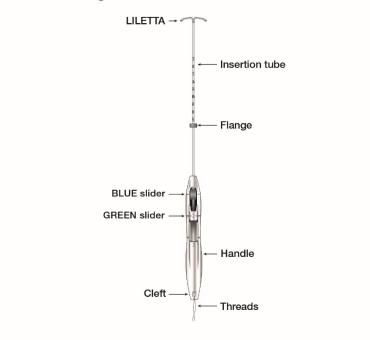
Inserter
The inserter device provided with LILETTA is a single-use, disposable, sterile insertion system (tube, flange, handle; Figure 16), partially preloaded with the IUS product for intrauterine administration.
Once LILETTA has been inserted, the inserter is discarded.
Figure 16: Diagram of Inserter
Mechanism of Action
The local mechanism by which continuously released LNG provides contraception has not been conclusively demonstrated. Studies of LNG-releasing IUSs suggest several mechanisms for pregnancy prevention: prevention of fertilization due to the thickening of the cervical mucus, which inhibits sperm passage through the cervix, and inhibition of sperm mobility and function (capacitation), and alteration of the endometrium.
What if I need birth control for more than 6 years?
Liletta must be removed after 6 years. Your healthcare professional can place a new Liletta during the same office visit if you choose to continue using Liletta.
What if I want to stop using Liletta?
Liletta is intended for use up to 6 years, but you can stop using Liletta at any time by asking your healthcare professional to remove it. You could become pregnant as soon as Liletta is removed, so you should use another method of birth control if you do not want to become pregnant. Talk to your healthcare professional about the best birth control methods for you, because your new method may need to be started 7 days before Liletta is removed to prevent pregnancy.
What if I change my mind about birth control and want to become pregnant in less than 6 years?
Your healthcare professional can remove Liletta at any time. You could become pregnant as soon as Liletta is removed. About 6 out of 7 women who want to become pregnant will become pregnant sometime in the first year after Liletta is removed.
How does Liletta work?
Liletta may work in several ways including thickening cervical mucus, inhibiting sperm movement, reducing sperm survival, and thinning the lining of your uterus. It is not known exactly how these actions work together to prevent pregnancy.

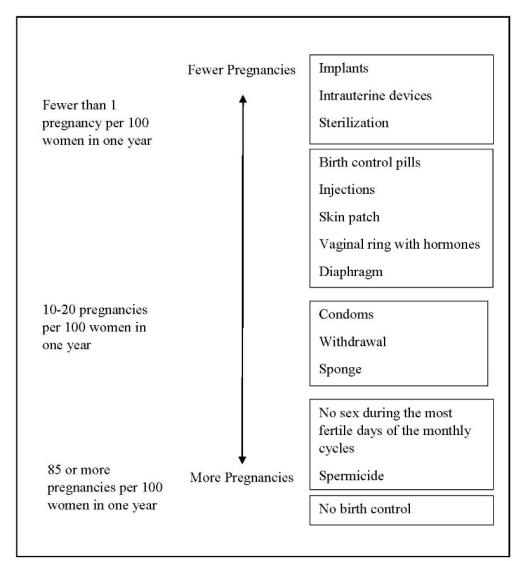
How well does Liletta work for contraception?
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
Liletta, an intrauterine system (IUS), is also known as an intrauterine device (IUD), which is described in the box at the top of the chart.

Who might use Liletta?
You might choose Liletta if you:
- want long-term birth control that provides a low chance of getting pregnant (less than 1 in 100)
- want birth control that works continuously for up to 6 years
- want birth control that is reversible
- want a birth control method that you do not need to take daily
- are willing to use a birth control method that is placed in the uterus
- want birth control that does not contain estrogen
What is the most important information I should know about Liletta?
Liletta does not protect against HIV infection (AIDS) and other sexually transmitted infections (STIs).
Who should not use Liletta?
Do not use Liletta if you:
- are or might be pregnant; Liletta cannot be used as an emergency contraceptive
- have a serious pelvic infection called pelvic inflammatory disease (PID) or endometritis unless you have had a normal pregnancy after the infection went away
- have an untreated lower genital infection now
- have had an infection from an abortion within the last 3 months
- can get infections easily. For example, if you:
- have problems with your immune system
- have multiple sexual partners or your partner has multiple sexual partners
- use or abuse intravenous drugs
- have or suspect you might have cancer of the uterus or cervix
- have bleeding from the vagina that has not been explained
- have short-term (acute) liver disease or liver tumor
- have breast cancer or any other cancer that is sensitive to progestin (a female hormone), now or in the past
- have an intrauterine system in your uterus already
- have a condition of the uterus that changes the shape of the uterine cavity, such as large fibroid tumors
- are allergic to levonorgestrel, silicone, polyethylene, or barium sulfate
What should I tell my healthcare provider before using Liletta?
Before having Liletta placed, tell your healthcare professional if you have or have had any medical conditions, including those listed below:
- any of the conditions listed above
- a heart attack
- a stroke
- been born with heart disease or have problems with your heart valves
- problems with blood clotting or take medicine to reduce clotting
- high blood pressure
- recently had a baby or if you are breastfeeding
- severe migraine headaches
- severe or frequent headaches
- AIDS, HIV, or any other sexually transmitted infection
Tell your healthcare professional about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Liletta?
Liletta is placed by your healthcare professional during an in-office visit.
First, your healthcare professional will examine your pelvis to find the exact position of your uterus. Your healthcare professional will then clean your vagina and cervix with an antiseptic solution and slide a plastic tube containing Liletta through the cervix into your uterus. Your healthcare professional will then remove the plastic tube and leave Liletta in your uterus. Your healthcare professional will trim the threads to the right length.
You may experience pain, bleeding, or dizziness during and after placement. If your symptoms do not pass within 30 minutes after placement, Liletta may not have been placed correctly. Your healthcare professional will examine you to see if Liletta needs to be removed or replaced.
What are the possible side effects of Liletta?
Liletta can cause serious side effects, including:
- ectopic pregnancy. If you get pregnant while using Liletta, you might have an ectopic pregnancy. This means that the pregnancy is not in the uterus. Unusual vaginal bleeding or abdominal pain especially with missed periods may be a sign of ectopic pregnancy. Ectopic pregnancy is a medical emergency that often requires surgery. Ectopic pregnancy can cause internal bleeding, infertility, and even death.
- intrauterine pregnancy risks. There are also risks if you get pregnant while using Liletta and the pregnancy is in the uterus. Severe infection, miscarriage, premature labor, premature delivery, and even death can occur with pregnancies that continue with an intrauterine system (IUS). Because of this, your healthcare professional may try to remove Liletta, even though removing it may cause a miscarriage. If Liletta cannot be removed, talk with your healthcare professional about the benefits and risks of continuing the pregnancy. If, after seeing your healthcare professional, you choose to continue your pregnancy, see your healthcare professional regularly. Call your healthcare professional right away if you get flu-like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge, or fluid leaking from your vagina. These may be signs of infection. It is not known if Liletta can cause long-term effects on the fetus if it stays in place during a pregnancy.
- life-threatening infection. Life-threatening infection can occur within the first few days after Liletta is placed. Call your healthcare professional immediately if you develop severe pain or fever shortly after Liletta is placed.
- pelvic inflammatory disease (PID) or endometritis. Some IUS users get a serious pelvic infection called pelvic inflammatory disease (PID) or endometritis. PID and endometritis are usually sexually transmitted. You have a higher chance of getting PID or endometritis if you or your partner has sex with other partners. PID or endometritis can cause serious problems such as infertility, ectopic pregnancy or pelvic pain that does not go away. PID is usually treated with antibiotics. More serious cases of PID or endometritis may require surgery. Removal of the uterus (hysterectomy) is sometimes needed. In rare cases, infections that start as PID or endometritis can even cause death.
Tell your healthcare professional right away if you have any of these signs of PID or endometritis: long-lasting or heavy bleeding, unusual vaginal discharge, low abdominal pain, painful sex, chills, or fever.
- perforation. Liletta may partially go into the wall of the uterus (become embedded) or go completely through the wall of the uterus (perforate). If this occurs, Liletta may no longer prevent pregnancy. If perforation occurs, Liletta may move outside the uterus and can cause internal scarring, infection, or damage to other organs. You may need surgery to have Liletta removed if it is embedded or perforation occurs. The risk of perforation is increased in breastfeeding women.
- expulsion. Liletta may come out of your uterus (expulsion). Expulsion occurs in about 4 out of 100 women, most often in the first year of use. You may become pregnant if Liletta comes out. If you think that Liletta has come out, use another birth control method like condoms and spermicide or do not have sex (vaginal intercourse) until you are seen by a healthcare professional.
- cysts on the ovary. Some women using Liletta develop a painful cyst on the ovary. These cysts usually disappear on their own in 2 to 3 months. However, a cyst can cause pain and sometimes cysts will need surgery.
- changes in bleeding. You may have bleeding and spotting between menstrual periods, especially during the first 3 to 6 months. Sometimes the bleeding is heavier than usual at first. However, the bleeding usually becomes lighter than usual and may be irregular. Call your healthcare professional if the bleeding remains heavier than usual or increases after it has been light for a while.
The most common side effects of Liletta include:
- vaginal bacterial infection
- yeast infection of the outer part of your vagina (vulvovaginal)
- acne
- headache
- nausea or vomiting
- pain during sex
- abdominal pain
- breast pain
- pelvic pain
- depression
- weight increase
- vaginal discharge
- mood changes
- anxiety
- back pain
- menstrual-like cramping
- Pain, bleeding, or dizziness during and after placement. If these symptoms do not stop within 30 minutes after placement, Liletta may not have been placed correctly, or they may be symptoms of perforation or expulsion. Your healthcare professional will examine you to see if Liletta needs to be removed or replaced.
- Missed menstrual periods. About 2 out of 10 women stop having periods after 1 year of Liletta use. If you do not have a period for 6 weeks during Liletta use, call your healthcare professional. If you have any concerns that you may be pregnant while using Liletta, do a urine pregnancy test and call your healthcare professional. When Liletta is removed, your menstrual periods will usually return to your previous pattern.
These are not all the possible side effects of Liletta. For more information, ask your healthcare professional or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the U.S. Food and Drug Administration (FDA) at 1-800-FDA-1088.
You may also report side effects to Allergan at 1-800-678-1605.
After Liletta has been inserted, when should I call my healthcare professional?
Call your healthcare professional if you have any concerns about Liletta. Be sure to call if you:
- think you are pregnant
- have pelvic pain or pain during sex
- have unusual vaginal discharge or genital sores
- have unexplained fever, flu-like symptoms or chills
- might be exposed to sexually transmitted infections (STIs)
- are concerned that Liletta may have been expelled (came out)
- cannot feel Liletta’s threads
- develop very severe or migraine headaches
- have yellowing of the skin or whites of the eyes. These may be signs of liver problems.
- have had a stroke or heart attack
- you or your partner becomes HIV positive
- have severe vaginal bleeding, bleeding that lasts a long time, or you miss your period
General information about the safe and effective use of Liletta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
You can ask your pharmacist or healthcare professional for information about Liletta that is written for health professionals.
For more information, go to www.LILETTA.com or call 1-855-LILETTA (1-855-545-3882).
How should I store Liletta?
Store at 20°C – 25°C (68°F – 77°F), with excursions permitted between 15°C – 30°C (59°F – 86°F) [See USP Controlled Room Temperature]. Store the sealed tray with peel-off lid in outer carton until use to protect from light.
What are the ingredients in Liletta?
Active ingredients: levonorgestrel
Inactive ingredients: dimethicone, barium sulfate
Label
PRINCIPAL DISPLAY PANEL