Lumoxiti
Generic name: moxetumomab pasudotox
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Lumoxiti?
Lumoxiti is a prescription medicine used to treat adults with hairy cell leukemia (HCL) that has come back or has not responded to previous treatment, and have received at least 2 other treatments, including a type of medicine called purine nucleoside analog (PNA).
It is not known if Lumoxiti is safe and effective in children.
Description
Moxetumomab pasudotox-tdfk is a CD22-directed cytotoxin. Moxetumomab pasudotox-tdfk is composed of a recombinant, murine immunoglobulin variable domain genetically fused to a truncated form of Pseudomonas exotoxin, PE38, that inhibits protein synthesis. Moxetumomab pasudotox-tdfk has an approximate molecular weight of 63 kDa and is produced in E. coli cells by recombinant DNA technology. During the moxetumomab pasudotox-tdfk manufacturing process, fermentation is carried out in nutrient medium containing the antibiotic kanamycin. However, kanamycin is cleared in the manufacturing process and is not detectable in the final product.
LUMOXITI (moxetumomab pasudotox-tdfk) for injection is supplied as a sterile, preservative-free, white to off-white lyophilized cake or powder in a single-dose vial for reconstitution and dilution prior to intravenous infusion. Each single-dose vial contains 1 mg moxetumomab pasudotox-tdfk, glycine (80 mg), polysorbate 80 (0.2 mg), sodium phosphate monobasic monohydrate (3.4 mg), sucrose (40 mg), and sodium hydroxide to adjust pH to 7.4. After reconstitution with 1.1 mL Sterile Water for Injection, USP, the resulting 1 mg/mL solution allows a withdrawal volume of 1 mL. Prior to intravenous infusion, the reconstituted vial(s) of solution are added to an infusion bag containing 50 mL of 0.9% Sodium Chloride Injection, USP and 1 mL of IV Solution Stabilizer.
IV Solution Stabilizer is a sterile, preservative-free, colorless to slightly yellow, clear solution free from visible particles and supplied in a single-dose vial. Each vial contains 1 mL solution. Each vial contains citric acid monohydrate (0.7 mg), polysorbate 80 (6.5 mg), sodium citrate dihydrate (6.4 mg), and Water for Injection, USP. The pH is 6.0.
The LUMOXITI and IV Solution Stabilizer vial stoppers are not made with natural rubber latex.
Mechanism of Action
Moxetumomab pasudotox-tdfk is a CD22-directed cytotoxin. Moxetumomab pasudotox-tdfk binds CD22 on the cell surface of B-cells and is internalized. Moxetumomab pasudotox-tdfk internalization results in ADP-ribosylation of elongation factor 2, inhibition of protein synthesis, and apoptotic cell death.
What is the most important information I should know about Lumoxiti?
Lumoxiti can cause serious side effects, including:
- Capillary Leak Syndrome (CLS). Lumoxiti can cause fluid to leak from small blood vessels into your body’s tissues. This condition is called “Capillary Leak Syndrome” (CLS). CLS can quickly cause you to have symptoms that may become life-threatening if not treated right away. Get emergency medical help right away if you develop any of the following symptoms of CLS:
- swelling of your face, arms, or legs
- fast weight gain (increase in 5.5 pounds from Day 1 of your current cycle)
- weakness or dizziness
- shortness of breath or trouble breathing
- cough
- low blood pressureYour healthcare provider will check your weight and blood pressure before you receive each dose of Lumoxiti and as needed during treatment.
- Hemolytic Uremic Syndrome (HUS). Hemolytic uremic syndrome is a condition that affects your blood cells and kidneys and may be life-threatening if not treated right away. Get emergency medical help right away if you develop any of the following symptoms of HUS
- decrease in the amount of urine or dark urine (tea-colored)
- unusual bleeding or bruising of your skin
- stomach pain
- vomiting
- fever
- feeling tired
- changes in mood or behavior
- confusion
- seizures
- shortness of breath
- fast heartbeatYour healthcare provider will do blood tests to check your blood cells and kidneys before you receive each dose of Lumoxiti and during treatment as recommended by your healthcare provider.
If you develop any of these symptoms of CLS or HUS, your healthcare provider may monitor you in the hospital.
Getting medical treatment right away may help keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during your treatment with Lumoxiti. Your healthcare provider may delay or completely stop treatment with Lumoxiti if you have severe side effects.
See “What are the possible side effects of Lumoxiti?” below for other side effects of Lumoxiti.
What should I tell my healthcare provider before using Lumoxiti?
Before you receive Lumoxiti, tell your healthcare provider about all your medical conditions, including if you:
- have had conditions that affect your blood and blood vessels called HUS or severe thrombotic microangiopathy (TMA)
- have kidney problems
- are pregnant or plan to become pregnant. Lumoxiti may harm your unborn baby.
- If you are a female who can become pregnant, you should use effective birth control during treatment with Lumoxiti and for at least 30 days after your last dose of Lumoxiti.
- If you are a female who can become pregnant, your healthcare provider will perform a pregnancy test before you start treatment with Lumoxiti.
- Tell your healthcare provider right away if you become pregnant during treatment with Lumoxiti.
- are breastfeeding or plan to breastfeed. It is not known if Lumoxiti passes into your breast milk. You and your healthcare provider should decide if you will receive Lumoxiti or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines with you and show it to your healthcare provider when you get a new medicine.
How should I use Lumoxiti?
- Your healthcare provider will give you Lumoxiti into your vein through an intravenous (IV) line over 30 minutes.
- Lumoxiti is usually given on Day 1, Day 3, and Day 5 of a 28-day treatment cycle. This is 1 treatment cycle. You may receive up to 6 treatment cycles.
- Your healthcare provider will give you medicines and IV fluids before and after your infusions.
- It is important for you to drink the additional prescribed amount of fluids (water, milk, or juice) of up to twelve 8‑oz glasses every 24 hours on Days 1 through 8 of each 28-day treatment cycle when you receive Lumoxiti infusions.
- Your healthcare provider will decide how many treatment cycles you need.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of Lumoxiti?
Lumoxiti can cause serious side effects, including:
- See “What is the most important information I should know about Lumoxiti?”
- Kidney problems. Lumoxiti may cause kidney problems. People who have HUS, are 65 years of age or older, or those who have kidney problems before starting treatment with Lumoxiti may have an increased risk of worse kidney problems after treatment with Lumoxiti. Tell your healthcare provider right away if you have any changes in the amount you urinate. Your healthcare provider will do tests to check your kidneys before you receive each dose of Lumoxiti and as needed during treatment. Your healthcare provider may delay your treatment with Lumoxiti if you have severe kidney problems.
- Infusion reactions. Lumoxiti can cause infusion reactions that are common but can also be serious. Infusion reactions may happen on the day you receive your Lumoxiti infusion. Signs and symptoms of infusion reactions may include:
- chills
- cough
- dizziness
- shortness of breath or wheezing
- feeling hot or flushing
- fast heartbeat
- headache
- changes in blood pressure
- muscle pain
- nausea
- fever
- vomitingYour healthcare provider may give you medicines to take before and after each Lumoxiti infusion.
- Electrolyte problems. Tell your healthcare provider if you get any of the following symptoms of electrolyte problems:
- muscle cramps
- numbness or tingling
- abnormal or fast heartbeat
- nausea
- seizuresYour healthcare provider will do blood tests to check your electrolytes before you receive each dose of Lumoxiti and during treatment as recommended by your healthcare provider.
The most common side effects of Lumoxiti include:
- swelling in your face, arms, or legs
- nausea
- feeling tired
- headache
- fever
- constipation
- low red blood cells (anemia)
- diarrhea
These are not all the possible side effects of Lumoxiti.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Lumoxiti
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Lumoxiti that is written for health professionals.
What are the ingredients in Lumoxiti?
Active ingredient: moxetumomab pasudotox-tdfk
Inactive ingredients: glycine, polysorbate 80, sodium phosphate monobasic monohydrate, sucrose, and sodium hydroxide
Inactive ingredients of IV Solution Stabilizer: citric acid monohydrate, polysorbate 80, sodium citrate dihydrate, Water for Injection, USP
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 0310-4715-11
- IV Solution Stabilizer for LUMOXITI® 1 mL
- Rx only
- Attention Pharmacist: Use ONLY to prepare the infusion bag prior to adding reconstituted LUMOXITI®, packaged separately.
- One single-dose vial. Discard unused portion.
- DO NOT use IV Solution Stabilizer to reconstitute LUMOXITI® vials
- Dosage: See LUMOXITI® prescribing information and te Healthcare Provider Instructions for Use for dosage, preparation and administration.
- Do not use if vial seal is broken or missing.
- Manufactured for: AstraZeneca AB, Södertälje, Sweden SE-15185
- U.S. License No. 2059
- Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850

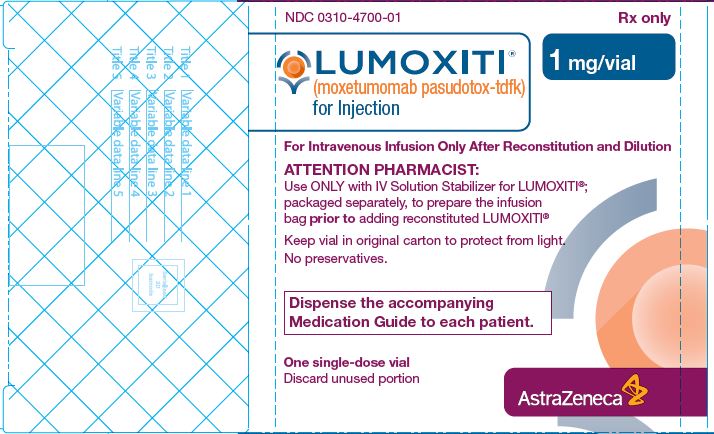
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 0310-4700-01
- LUMOXITI® (moxetumomab pasudotox-tdfk) for injection
- 1 mg/vial carton
- For Intravenous Infusion Only After Reconstitution and Dilution
- Rx only
- Attention Pharmacist: Use only with IV Solution Stabilizer for LUMOXITI®; packaged separately, to prepare the infusion bag prior to adding reconstituted LUMOXITI®
- Keep vial in original carton to protect from light.
- No preservatives
- Dispense the accompanying Medication Guide to each patient.
- One single-dose vial
- Discard unused portion
- Manufactured by: AstraZeneca AB, Södertälje, Sweden SE-15185
- U.S. License No. 2059
- Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
SRC: NLM .