Lexiva
Generic name: fosamprenavir
Drug class: Protease inhibitors
Medically reviewed by A Ras MD.
What is Lexiva?
Lexiva is a prescription medicine that is used together with other antiretroviral medicines to treat human immunodeficiency virus 1 (HIV-1) infection.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
It is not known if Lexiva is safe and effective in children younger than 4 weeks of age.
Description
LEXIVA (fosamprenavir calcium) is a prodrug of amprenavir, an inhibitor of HIV protease. The chemical name of fosamprenavir calcium is (3S)-tetrahydrofuran-3-yl (1S,2R)-3-[[(4-aminophenyl) sulfonyl](isobutyl)amino]-1-benzyl-2-(phosphonooxy) propylcarbamate monocalcium salt. Fosamprenavir calcium is a single stereoisomer with the (3S)(1S,2R) configuration. It has a molecular formula of C25H34CaN3O9PS and a molecular weight of 623.7. It has the following structural formula:
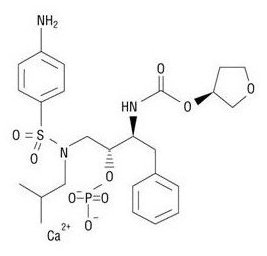
Fosamprenavir calcium is a white to cream-colored solid with a solubility of approximately 0.31 mg per mL in water at 25°C.
LEXIVA tablets are available for oral administration in a strength of 700 mg of fosamprenavir as fosamprenavir calcium (equivalent to approximately 600 mg of amprenavir). Each 700-mg tablet contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone K30. The tablet film-coating contains the inactive ingredients hypromellose, iron oxide red, titanium dioxide, and triacetin.
LEXIVA oral suspension is available in a strength of 50 mg per mL of fosamprenavir as fosamprenavir calcium equivalent to approximately 43 mg of amprenavir. LEXIVA oral suspension is a white to off-white suspension with a grape-bubblegum-peppermint flavor. Each one milliliter (1 mL) contains the inactive ingredients artificial grape-bubblegum flavor, calcium chloride dihydrate, hypromellose, methylparaben, natural peppermint flavor, polysorbate 80, propylene glycol, propylparaben, purified water, and sucralose.
What is the most important information I should know about Lexiva?
Lexiva can interact with other medicines and cause serious side effects. It is important to know the medicines that should not be taken with Lexiva. See the section “Who should not take Lexiva?”
Lexiva can cause serious side effects, including:
- Severe skin reactions. Lexiva may cause severe or life-threatening skin reactions or rash.
If you get a rash with any of the following symptoms, stop taking Lexiva and call your healthcare provider or get medical help right away:- hives or sores in your mouth, or your skin blisters and peels
- trouble swallowing or breathing
- swelling of your face, eyes, lips, tongue, or throat
For more information about side effects, see “What are the possible side effects of Lexiva?”
Who should not take Lexiva?
Do not take Lexiva if you:
- are allergic to amprenavir, fosamprenavir calcium, or any of the ingredients in Lexiva. See the end of this leaflet for a complete list of ingredients in Lexiva.
- take any of the following medicines:
- alfuzosin
- rifampin
- ergot including:
- dihydroergotamine mesylate
- ergonovine
- ergotamine tartrate
- methylergonovine
- cisapride
- St. John’s wort (Hypericum perforatum)
- lomitapide
- lovastatin
- simvastatin
- pimozide
- delavirdine mesylate
- sildenafil (Revatio), for treatment of pulmonary arterial hypertension
- triazolam
- midazolam, when taken by mouthSerious problems can happen if you or your child take any of the medicines listed above with Lexiva.
If you are taking Lexiva with ritonavir, do not take the following medicines:
- flecainide
- propafenone
- lurasidone
What should I tell my healthcare provider before taking Lexiva?
Before taking Lexiva, tell your healthcare provider about all of your medical conditions, including if you:
- are allergic to medicines that contain sulfa.
- have or have had liver problems, including hepatitis B or C virus infection.
- have kidney problems.
- have high blood sugar (diabetes).
- have hemophilia.
- have high cholesterol.
- are pregnant or plan to become pregnant. It is not known if Lexiva will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant during treatment with Lexiva.Some medicines interact with Lexiva. Keep a list of your medicines to show your healthcare provider and pharmacist.
- Lexiva may reduce how well hormonal contraceptives (birth control pills) work. Females who may become pregnant should use a different form of birth control or an additional barrier method of birth control during treatment with Lexiva.Pregnancy Registry. There is a pregnancy registry for women who take Lexiva during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Lexiva.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if Lexiva can pass to your baby in your breast milk.
- Talk with your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines interact with Lexiva. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Lexiva.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Lexiva with other medicines.
How should I take Lexiva?
- Take Lexiva exactly as your healthcare provider tells you to take it.
- If you miss a dose of Lexiva, take it as soon as you remember. Do not take 2 doses at the same time or take more than your healthcare provider tells you to take.
- Stay under the care of a healthcare provider during treatment with Lexiva.
- If your child is taking Lexiva, your child’s healthcare provider will decide the right dose based on your child’s weight.
- Lexiva tablets may be taken with or without food.
- Adults should take Lexiva oral suspension without food.
- Children should take Lexiva oral suspension with food. If your child vomits within 30 minutes after taking a dose of Lexiva, the dose should be repeated.
- Shake Lexiva oral suspension well before each use.
- Do not run out of Lexiva. The virus in your blood may increase and the virus may become harder to treat. When your supply starts to run low, get more from your healthcare provider or pharmacy.
- If you take too much Lexiva, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Lexiva?
Lexiva may cause serious side effects including:
- See “What is the most important information I should know about Lexiva?”
- Liver problems. Your healthcare provider should do blood tests before and during your treatment with Lexiva to check your liver function. Some people with liver problems, including hepatitis B or C, may have an increased risk of developing worsening liver problems during treatment with Lexiva.
- Diabetes and high blood sugar (hyperglycemia). Some people who take protease inhibitors, including Lexiva, can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or urinate often during treatment with Lexiva.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Call your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- Increase in body fat. An increase in body fat can happen in people who take protease inhibitors, including Lexiva. The exact cause and long-term health effects of these conditions are not known.
- Changes in blood tests. Some people have changes in blood tests while taking Lexiva. These include an increase in liver function tests, blood fat levels (cholesterol and triglycerides) and decrease in red blood cells. Your healthcare provider should do regular blood tests before and during your treatment with Lexiva.
- Increased bleeding problems in some people with hemophilia. Some people with hemophilia have increased bleeding with protease inhibitors, including Lexiva.
- Kidney stones. Some people have developed kidney stones during treatment with Lexiva. Tell your healthcare provider right away if you develop any of the following signs or symptoms of kidney stones:
- pain in your side
- blood in your urine
- pain when you urinate
The most common side effects of Lexiva in adults include:
- nausea
- vomiting
- rash
- diarrhea
- headache
The most common side effects of Lexiva in children include vomiting and decrease in white blood cells.
These are not all the possible side effects of Lexiva.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Lexiva
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Lexiva for a condition for which it was not prescribed. Do not give Lexiva to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Lexiva that is written for health professionals.
How should I store Lexiva?
- Store Lexiva tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the bottle of Lexiva tablets tightly closed.
- Store Lexiva oral suspension at room temperature or in the refrigerator between 41°F to 86°F (5°C to 30°C). Refrigeration of Lexiva oral suspension may improve taste for some people.
- Do not freeze.
- Lexiva comes in a child-resistant package.
Keep Lexiva and all medicines out of the reach of children.
What are the ingredients in Lexiva?
Active ingredient: fosamprenavir calcium
Inactive ingredients:
Tablets: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone K30. The tablet film-coating contains the inactive ingredients hypromellose, iron oxide red, titanium dioxide, and triacetin.
Oral Suspension: artificial grape-bubblegum flavor, calcium chloride dihydrate, hypromellose, methylparaben, natural peppermint flavor, polysorbate 80, propylene glycol, propylparaben, purified water, and sucralose.
Label
PRINCIPAL DISPLAY PANEL
- NDC 49702-208-53
- LEXIVA
- (fosamprenavir calcium)
- Oral Suspension
- 50 mg/mL
- ViiV Healthcare
- SHAKE VIGOROUSLY BEFORE USING.
- ALERT: Find out about medicines that should NOT be taken with LEXIVA.
- 225 mL
- Rx only
- Made in Canada
- ©2020 ViiV Healthcare group of companies or its licensor.
- 62000000056125 Rev. 10/20
-

PRINCIPAL DISPLAY PANEL
- NDC 49702-207-18
- LEXIVA
- (fosamprenavir calcium)
- TABLETS
- 700 mg
- 60 Tablets
- ALERT: Find out about medicines that should NOT be taken with LEXIVA.
- Note to Pharmacist: Do not cover ALERT box with pharmacy label.
- Rx only
- For use in combination regimens with or without ritonavir.
- Each tablet contains 700 mg of fosamprenavir as fosamprenavir calcium.
- This package is child-resistant. Keep out of reach of children.
- Store at controlled room temperature of 25˚C (77˚F); excursions permitted to 15˚ to 30˚C (59˚ to 86˚F) (see USP Controlled Room Temperature).
- Keep bottle tightly closed.
- See prescribing information for dosage information.
- Trademarks owned or licensed by ViiV Healthcare.
- ©2020 ViiV Healthcare or licensor.
- Manufactured for:
- ViiV Healthcare
- Research Triangle Park, NC 27709
- Made in Belgium
- Rev. 11/20
- 62000000055505

SRC: NLM .