Mavyret
Generic name: glecaprevir and pibrentasvir
Drug class: Antiviral combinations
Medically reviewed by A Ras MD.
What is Mavyret?
Mavyret is a prescription medicine used to treat adults and children 12 years of age and older or weighing at least 99 pounds (45 kilograms) with chronic (lasting a long time) hepatitis C virus (HCV) genotypes 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis.
HCV genotype 1 infection and have been previously treated with a regimen that contained an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both.
Mavyret contains the two medicines: glecaprevir and pibrentasvir.
It is not known if Mavyret is safe and effective in children under 12 years of age.
Description
MAVYRET contains glecaprevir, a HCV NS3/4A PI, and pibrentasvir a HCV NS5A inhibitor. MAVYRET is available as a fixed dose combination tablet or coated pellets in unit-dose packets for oral administration.
Glecaprevir/Pibrentasvir Film-Coated Immediate Release Tablets
Each tablet contains 100 mg of glecaprevir and 40 mg of pibrentasvir. Glecaprevir and pibrentasvir are presented as a co-formulated, fixed-dose combination, immediate release bilayer tablet.
The tablet contains the following inactive ingredients: colloidal silicon dioxide, copovidone (type K 28), croscarmellose sodium, hypromellose 2910, iron oxide red, lactose monohydrate, polyethylene glycol 3350, propylene glycol monocaprylate (type II), sodium stearyl fumarate, titanium dioxide, and vitamin E (tocopherol) polyethylene glycol succinate.
The tablets do not contain gluten.
Glecaprevir/Pibrentasvir Coated Oral Pellets
MAVYRET oral pellets are small, pink and yellow and supplied in unit-dose packets for oral administration. Each unit-dose of MAVYRET oral pellets in packets contains 50 mg glecaprevir and 20 mg pibrentasvir and the following inactive ingredients: colloidal silicon dioxide, copovidone (type K 28), croscarmellose sodium, hypromellose 2910, iron oxide red, iron oxide yellow, lactose monohydrate, polyethylene glycol/macrogol 3350, propylene glycol monocaprylate (type II), sodium stearyl fumarate, titanium dioxide, vitamin E (tocopherol) polyethylene glycol succinate.
The oral pellets do not contain gluten.
Glecaprevir drug substance:
The chemical name of glecaprevir is (3aR,7S,10S,12R,21E,24aR)-7-tert-butyl-N-{(1R,2R)-2-(difluoromethyl)-1-[(1-methylcyclopropane-1-sulfonyl)carbamoyl]cyclopropyl}-20,20-difluoro-5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1H,10H-9,12-methanocyclopenta[18,19][1,10,17,3,6]trioxadiazacyclononadecino[11,12-b]quinoxaline-10-carboxamide hydrate.
The molecular formula is C38H46F4N6O9S (anhydrate) and the molecular weight for the drug substance is 838.87 g/mol (anhydrate). The strength of glecaprevir is based on anhydrous glecaprevir. Glecaprevir is a white to off-white crystalline powder with a solubility of less than 0.1 to 0.3 mg/mL across a pH range of 2–7 at 37°C and is practically insoluble in water, but is sparingly soluble in ethanol. Glecaprevir has the following molecular structure:
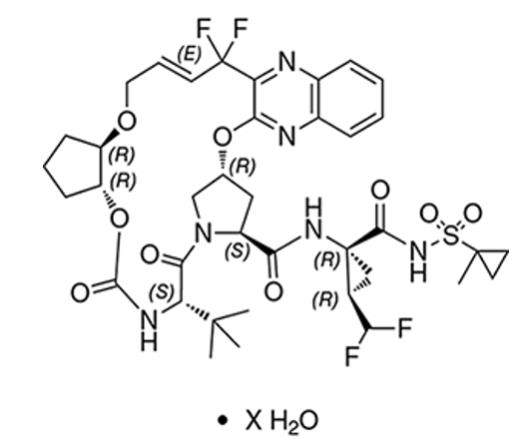
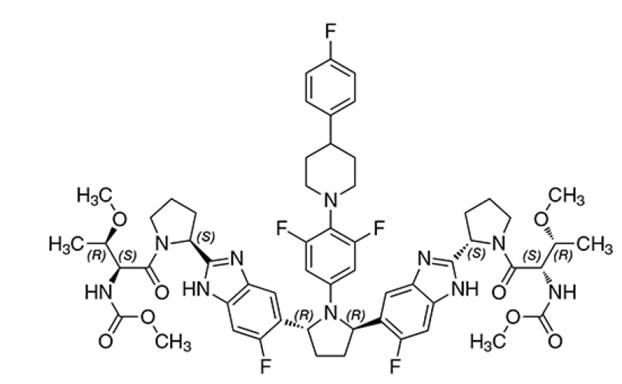
Pibrentasvir drug substance:
The chemical name of pibrentasvir is Methyl {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}-5-(6-fluoro-2-{(2S)-1-[N-(methoxycarbonyl)-O-methyl-L-threonyl]pyrrolidin-2-yl}-1H-benzimidazol-5-yl)pyrrolidin-2-yl]-6-fluoro-1H-benzimidazol-2-yl}pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl}carbamate.
The molecular formula is C57H65F5N10O8 and the molecular weight for the drug substance is 1113.18 g/mol. Pibrentasvir is a white to off-white to light yellow crystalline powder with a solubility of less than 0.1 mg/mL across a pH range of 1–7 at 37°C and is practically insoluble in water, but is freely soluble in ethanol. Pibrentasvir has the following molecular structure:
Mechanism of Action
MAVYRET is a fixed-dose combination of glecaprevir and pibrentasvir, which are direct-acting antiviral agents against the hepatitis C virus
What is the most important information I should know about Mavyret?
Mavyret can cause serious side effects, including:
Hepatitis B virus reactivation. Before starting treatment with Mavyret, your healthcare provider will do blood tests to check for hepatitis B virus infection. If you have ever had hepatitis B virus infection, the hepatitis B virus could become active again during or after treatment for hepatitis C virus with Mavyret. Hepatitis B virus that becomes active again (called reactivation) may cause serious liver problems including liver failure and death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop taking Mavyret.
For more information about side effects, see the section “What are the possible side effects of Mavyret?”
Who should not take Mavyret?
Do not take Mavyret if you
- have certain liver problems
- also take any of the following medicines:
- atazanavir
- rifampin
What should I tell my healthcare provider before taking Mavyret?
Before taking Mavyret, tell your healthcare provider about all of your medical conditions, including if you:
- have had hepatitis B virus infection
- have liver problems other than hepatitis C virus infection.
- have HIV-1 infection
- have had a liver or a kidney transplant
- are pregnant or plan to become pregnant. It is not known if Mavyret will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Mavyret passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Mavyret.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Mavyret and other medicines may affect each other. This can cause you to have too much or not enough Mavyret or other medicines in your body. This may affect the way Mavyret or your other medicines work or may cause side effects.
Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Mavyret.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Mavyret with other medicines.
How should I take Mavyret?
- Take Mavyret exactly as your healthcare provider tells you to take it. Do not change your dose unless your healthcare provider tells you to.
- Do not stop taking Mavyret without first talking with your healthcare provider.
- Take 3 Mavyret tablets at one time each day.
- Take Mavyret with food.
- It is important that you do not miss or skip doses of Mavyret during treatment.
- If you miss a dose of Mavyret and it is:
- Less than 18 hours from the time you usually take Mavyret, take the missed dose with food as soon as possible. Then take your next dose at your usual time.
- More than 18 hours from the time you usually take Mavyret, do not take the missed dose. Take your next dose as usual with food.
- If you take too much Mavyret, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Mavyret?
Mavyret can cause serious side effects, including:
- Hepatitis B virus reactivation. See “What is the most important information I should know about Mavyret?”
- In people who had or have advanced liver problems before starting treatment with Mavyret: rare risk of worsening liver problems, liver failure and death. Your healthcare provider will check you for signs and symptoms of worsening liver problems during treatment with Mavyret. Tell your healthcare provider right away if you have any of the following signs and symptoms:
- nausea
- tiredness
- yellowing of your skin or white part of your eyes
- bleeding or bruising more easily than normal
- confusion
- dark, black, or bloody stool
- loss of appetite
- diarrhea
- dark or brown (tea-colored) urine
- swelling or pain on the upper right side of your stomach area (abdomen)
- sleepiness
- vomiting of blood
- lightheadedness
The most common side effects of Mavyret include headache and tiredness.
These are not all the possible side effects of Mavyret.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Mavyret
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Mavyret for a condition for which it was not prescribed. Do not give Mavyret to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Mavyret that is written for health professionals.
How should I store Mavyret?
- Store Mavyret at or below 86°F (30°C).
- Keep Mavyret in its original blister package until you are ready to take it.
Keep Mavyret and all medicines out of the reach of children.
What are the ingredients in Mavyret?
Active ingredients: glecaprevir and pibrentasvir
Inactive ingredients: colloidal silicon dioxide, copovidone (type K 28), croscarmellose sodium, hypromellose 2910, iron oxide red, lactose monohydrate, polyethylene glycol 3350, propylene glycol monocaprylate (type II), sodium stearyl fumarate, titanium dioxide, and vitamin E (tocopherol) polyethylene glycol succinate. The tablets do not contain gluten.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0074-2625-80
- Rx only
- MAVYRET®
- glecaprevir/pibrentasvir tablets
- 100 mg/40 mg
- For Institutional Use Only84 Tablets
- abbvie
- Do not accept if seal over bottle opening is broken or missing.
- Each tablet contains glecaprevir and pibrentasvir 100 mg/40 mg.
- Store at or below 30°C (86°F).
- See package insert for full Prescribing Information.
- AbbVie Inc.
- North Chicago, IL 60064 USA
- See opposite side of label for Customs Country of Origin.
- ©2020 AbbVie Inc.
SRC: NLM .
