Nitro-Dur
Generic name: nitroglycerin (transdermal)
Brand names: Minitran, Nitro TD Patch-A, Nitro-Dur
Drug classes: Antianginal agents, Vasodilators
Medically reviewed by A Ras MD.
What is Nitro-Dur?
Nitro-Dur is a unique method of administering nitroglycerin to the bloodstream. Nitro-Dur eliminates the swallowing of pills or the application of a messy ointment. Nitroglycerin is a medication your doctor has prescribed for you to help reduce the frequency and severity of angina attacks (chest pain).
Description
Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:
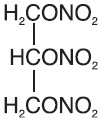
and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.
The NITRO-DUR ® (nitroglycerin) Transdermal Infusion System is a flat unit designed to provide continuous controlled release of nitroglycerin through intact skin. The rate of release of nitroglycerin is linearly dependent upon the area of the applied system; each cm 2 of applied system delivers approximately 0.02 mg of nitroglycerin per hour. Thus, the 5-,10-, 15-, 20-, 30-, and 40-cm2 systems deliver approximately 0.1, 0.2, 0.3, 0.4, 0.6, and 0.8 mg of nitroglycerin per hour, respectively.
The remainder of the nitroglycerin in each system serves as a reservoir and is not delivered in normal use. After 12 hours, for example, each system has delivered approximately 6% of its original content of nitroglycerin.
The NITRO-DUR transdermal system contains nitroglycerin in acrylic-based polymer adhesives with a resinous cross-linking agent to provide a continuous source of active ingredient. Each unit is sealed in a paper polyethylene-foil pouch.
Cross section of the system.
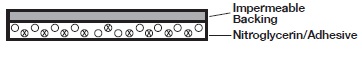
How your Nito-Dur Transdermal Infusion System works
Nitroglycerin causes the veins (vessels that return blood to the heart) to relax so that the work load of the heart is reduced. This lowers the heart’s oxygen needs.
As a result, the heart muscle is well nourished and the frequency of angina attacks is reduced. Nitro-Dur is applied directly to the skin. The nitroglycerin passes from the adhesive surface through the skin—allowing medication to be absorbed directly into the bloodstream. This manner of delivering medicine to your bloodstream provides you with nitroglycerin with one daily application of a Nitro-Dur unit.
How should I use Nitro-Dur?
- See the Instructions for use that come with Nitro-Dur.
- Allow Nitro-Dur to stay in place as directed by your doctor.
- Showering is permitted with Nitro-Dur in place.
- Nitro-Dur is boxed so that you have a 30-day supply. Be sure to check your supply periodically. Before it runs low, you should visit your pharmacist for a refill or ask your doctor to renew your Nitro-Dur prescription.
- It is important that you do not miss a day of your Nitro-Dur therapy. If your schedule needs to be changed, your doctor will give you special instructions.
- Nitro-Dur has been prescribed for you. Do not give your medication to anyone else.
- Nitro-Dur is for prevention of angina; not for treatment of an acute angina attack.
- Notify your doctor if angina attacks change for the worse.
Cautions
If your doctor has prescribed “under-the-tongue” nitroglycerin tablets in addition to Nitro-Dur, you should sit down before taking the “under-the-tongue” tablet. If dizziness should occur, notify your doctor. This may be an indication that the “under-the-tongue” tablet dosage needs to be reduced.
What are the possible side effects of Nitro-Dur?
The most common side effect experienced by people taking nitroglycerin is headache. Your doctor may tell you to take a mild analgesic to relieve the headache.
Some people may experience dizziness. This is due to a slight decrease in blood pressure, which is usually experienced when a person changes position, from lying flat to sitting upright or from sitting to standing. If this occurs, sit down until the dizziness stops, then notify your doctor.
He or she may wish to reduce your Nitro-Dur dosage. In some people, nitroglycerin preparations may cause the skin to feel flushed or the heart to beat faster. If this should occur, notify your doctor; again, he or she may wish to change your Nitro-Dur dosage.
Nitro-Dur is a unique drug that depends on direct contact with the skin to work. For this reason, the skin should be reasonably hair-free, clean, and dry
How should I store Nitro-Dur?
- Store at room temperature 77°F (25°C).
- Nitro-Dur should be kept out of reach of children and pets.
What are the ingredients in Nitro-Dur?
Active ingredient: Nitroglycerin
Instructions for use for Nitro-Dur
Placement area
Select a reasonably hair-free application site. Avoid extremities below the knee or elbow, skin folds, scar tissue, burned or irritated areas.

Application
Wash hands before applying.

Hold the unit with brown lines facing you, in an up and down position.

Bend the sides of unit away from you, then toward you until you hear the “SNAP”.

Peel off one side of the plastic backing.

Using the other half of the backing as a handle, apply the sticky side of the patch to the skin.

Press the sticky side on the skin, and smooth down.

Fold back the remaining side of the patch. Grasp the edge of the plastic applicator by the stripe, and pull it across the skin.

Wash hands to remove any drug.
Removal

Press down on the center of the system to raise its outer edge away from the skin.

Grasp the edge gently, and slowly peel the unit away from skin. Wash skin area with soap and water. Towel dry. Wash hands. You may use a different application site everyday.
Skin care
- After you remove Nitro-Dur, your skin may feel warm and appear red. This is normal. The redness will disappear in a short time. If the area feels dry, you may apply a soothing lotion.
- Any redness or rash that does not disappear should be called to your doctor’s attention.
Label
PRINCIPAL DISPLAY PANEL – 20 MG POUCH BOX
- NDC 50742-513-30
Contents: 30 units
Nitro-Dur ®
(nitroglycerin)
Transdermal Infusion System
0.1 mg/hr (5 cm 2)
Each unit contains 20 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.1 mg/hr.
Rx only

PRINCIPAL DISPLAY PANEL – 40 MG POUCH BOX
NDC 50742-514-30
Contents: 30 units
Nitro-Dur ®
(nitroglycerin)
Transdermal Infusion System
0.2 mg/hr (10 cm2)
Each unit contains 40 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.2 mg/hr.
Rx only

PRINCIPAL DISPLAY PANEL – 60 MG POUCH BOX
NDC 50742-515-30
Contents: 30 units
Nitro-Dur ®
(nitroglycerin)
Transdermal Infusion System
0.3 mg/hr (15 cm 2)
Each unit contains 60 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.3 mg/hr.
Rx only

PRINCIPAL DISPLAY PANEL – 80 MG POUCH BOX
NDC 50742-516-30
Contents: 30 units
Nitro-Dur ®
(nitroglycerin)
Transdermal Infusion System
0.4 mg/hr (20 cm 2)
Each unit contains 80 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.4 mg/hr.
Rx only

SRC: NLM .