Tabrecta
Generic name: capmatinib (tablets)
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Tabrecta?
Tabrecta is a prescription medicine used to treat adults with a kind of lung cancer called non-small cell lung cancer (NSCLC) that has spread to other parts of the body or cannot be removed by surgery (metastatic), and whose tumors have an abnormal mesenchymal epithelial transition (MET) gene
It is not known if Tabrecta is safe and effective in children.
Description
Capmatinib is a kinase inhibitor. The chemical name is 2-Fluoro-N-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide—hydrogen chloride—water (1/2/1). The molecular formula for capmatinib hydrochloride is C23H21Cl2FN6O2. The relative molecular mass is 503.36 g/mol for the hydrochloride salt and 412.43 g/mol for the free base.
Chemical structure for capmatinib hydrochloride
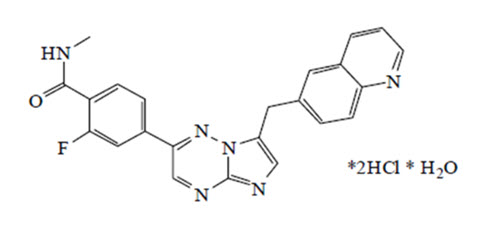
Capmatinib hydrochloride is a yellow powder with a pKa1 of 0.9 (calculated) and pKa2 of 4.5 (experimentally). Capmatinib hydrochloride is slightly soluble in acidic aqueous solutions at pH 1 and 2 and of further decreasing solubility towards neutral condition. The log of the distribution coefficient (n-octanol/acetate buffer pH 4.0) is 1.2.
TABRECTA is supplied for oral use as ovaloid, curved film-coated tablets with beveled edges, unscored containing 150 mg (pale orange brown color) or 200 mg (yellow color) capmatinib (equivalent to 176.55 mg or 235.40 mg respectively of capmatinib hydrochloride anhydrous). Each tablet strength contains colloidal silicon dioxide; crospovidone; magnesium stearate; mannitol; microcrystalline cellulose; povidone; and sodium lauryl sulfate as inactive ingredients.
The 150 mg tablet coating contains ferric oxide, red; ferric oxide, yellow; ferrosoferric oxide; hypromellose; polyethylene glycol (PEG) 4000; talc; and titanium dioxide. The 200 mg tablet coating contains ferric oxide, yellow; hypromellose; polyethylene glycol (PEG) 4000; talc; and titanium dioxide.
Mechanism of Action
Capmatinib is a kinase inhibitor that targets MET, including the mutant variant produced by exon 14 skipping. MET exon 14 skipping results in a protein with a missing regulatory domain that reduces its negative regulation leading to increased downstream MET signaling. Capmatinib inhibited cancer cell growth driven by a mutant MET variant lacking exon 14 at clinically achievable concentrations and demonstrated anti-tumor activity in murine tumor xenograft models derived from human lung tumors with either a mutation leading to MET exon 14 skipping or MET amplification. Capmatinib inhibited the phosphorylation of MET triggered by binding of hepatocyte growth factor or by MET amplification, as well as MET-mediated phosphorylation of downstream signaling proteins and proliferation and survival of MET-dependent cancer cells.
What should I tell my healthcare provider before taking Tabrecta?
Before taking Tabrecta, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had lung or breathing problems other than your lung cancer
- have or have had liver problems
- are pregnant or plan to become pregnant. Tabrecta can harm your unborn baby.
- Females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start your treatment with Tabrecta.
- You should use effective birth control during treatment and for 1 week after your last dose of Tabrecta. Talk to your healthcare provider about birth control choices that might be right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Tabrecta.
- Males who have female partners who can become pregnant:
- You should use effective birth control during treatment and for 1 week after your last dose of Tabrecta.
- Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if Tabrecta passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose of Tabrecta.
Tell your healthcare provider about all the medicines you take or start taking, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Tabrecta?
- Take Tabrecta exactly as your healthcare provider tells you.
- Take Tabrecta 2 times a day with or without food.
- Swallow Tabrecta tablets whole. Do not break, chew, or crush Tabrecta tablets.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Tabrecta if you have certain side effects.
- Do not change your dose or stop taking Tabrecta unless your healthcare provider tells you to.
- If you miss or vomit a dose of Tabrecta, do not make up the dose. Take your next dose at your regular scheduled time.
What should I avoid while taking Tabrecta?
- Your skin may be sensitive to the sun (photosensitivity) during treatment with Tabrecta. Use sunscreen or wear clothes that cover your skin during your treatment with Tabrecta to limit direct sunlight exposure.
What are the possible side effects of Tabrecta?
Tabrecta may cause serious side effects, including:
- lung or breathing problems. Tabrecta may cause inflammation of the lungs that can cause death. Tell your healthcare provider right away if you develop any new or worsening symptoms, including:
- cough
- fever
- trouble breathing or shortness of breath
Your healthcare provider may temporarily stop or permanently stop treatment with Tabrecta if you develop lung or breathing problems during treatment.
- liver problems. Tabrecta may cause abnormal liver blood test results. Your healthcare provider will do blood tests to check your liver function before you start treatment and during treatment with Tabrecta. Tell your healthcare provider right away if you develop any signs and symptoms of liver problems, including:
- your skin or the white part of your eyes turns yellow (jaundice)
- dark or “tea-colored” urine
- light-colored stools (bowel movements)
- confusion
- tiredness
- loss of appetite for several days or longer
- nausea and vomiting
- pain, aching, or tenderness on the right side of your stomach-area (abdomen)
- weakness
- swelling in your stomach-area
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Tabrecta if you develop liver problems during treatment.
- risk of sensitivity to sunlight (photosensitivity). See “What should I avoid while taking Tabrecta?”
The most common side effects of Tabrecta include:
- swelling of your hands or feet
- nausea
- tiredness and weakness
- vomiting
- loss of appetite
- changes in certain blood tests
These are not all of the possible side effects of Tabrecta. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tabrecta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Tabrecta for a condition for which it was not prescribed. Do not give Tabrecta to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tabrecta that is written for health professionals.
How should I store Tabrecta?
- Store Tabrecta at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Tabrecta in the original package with the drying agent (desiccant) cartridge.
- Protect Tabrecta from moisture.
- Throw away (discard) any unused Tabrecta you have left after 6 weeks of first opening the bottle.
Keep Tabrecta and all medicines out of the reach of children.
Label
PRINCIPAL DISPLAY PANEL
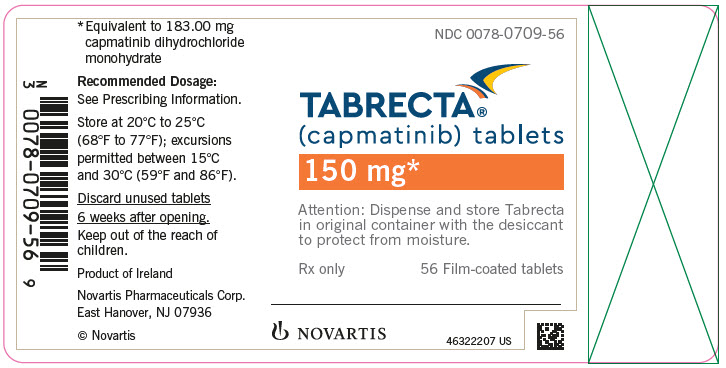
NDC 0078-0709-56
TABRECTA™
(capmatinib) tablets
150 mg*
Attention: Dispense and store Tabrecta
in original container with the desiccant
to protect from moisture.
Rx only
56 Film-coated tablets
NOVARTIS

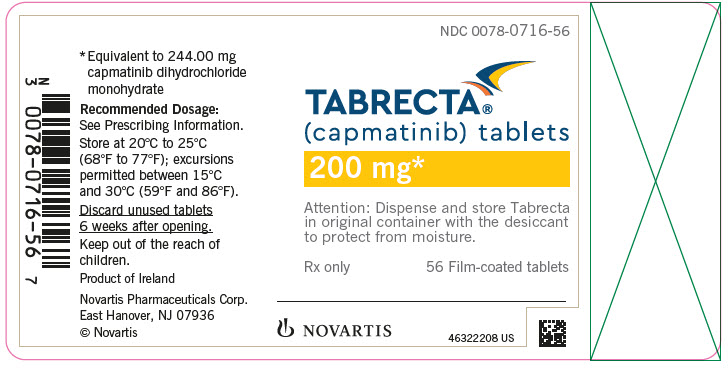
NDC 0078-0716-56
TABRECTA™
(capmatinib) tablets
200 mg*
Attention: Dispense and store Tabrecta
in original container with the desiccant
to protect from moisture.
Rx only
56 Film-coated tablets
NOVARTIS

What are the ingredients in Tabrecta?
Active ingredient: capmatinib
Inactive ingredients: Tablet core: colloidal silicon dioxide; crospovidone; magnesium stearate; mannitol; microcrystalline cellulose; povidone; and sodium lauryl sulfate.
Tablet coating (150 mg): ferric oxide, red; ferric oxide, yellow; ferrosoferric oxide; hypromellose; polyethylene glycol (PEG) 4000; talc; and titanium dioxide.
Tablet coating (200 mg): ferric oxide, yellow; hypromellose; polyethylene glycol (PEG) 4000; talc; and titanium dioxide.
SRC: NLM .