Tafamidis
Generic name: tafamidis
Brand names: Vyndamax, Vyndaqel
Dosage form: oral capsule (61 mg; meglumine 20 mg)
Drug class: Transthyretin stabilizers
Medically reviewed by A Ras MD.
What is tafamidis used for?
Tafamidis is a prescription medicine that is used to treat a type of heart problem (cardiomyopathy).
Description
VYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) contain tafamidis as the active moiety, which is a selective stabilizer of transthyretin.
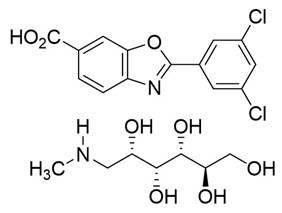
The chemical name of tafamidis meglumine is 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid mono (1-deoxy-1-methylamino-D-glucitol). The molecular formula is C14H7Cl2NO3 C7H17NO5, and the molecular weight is 503.33 g/mol.
Structural formula
Tafamidis meglumine 20-mg soft gelatin capsule for oral use contains a white to pink colored suspension of tafamidis meglumine 20 mg (equivalent to 12.2 mg of tafamidis free acid), and the following inactive ingredients: ammonium hydroxide 28%, brilliant blue FCF, carmine, gelatin, glycerin, iron oxide (yellow), polyethylene glycol 400, polysorbate 80, polyvinyl acetate phthalate, propylene glycol, sorbitan monooleate, sorbitol, and titanium dioxide.
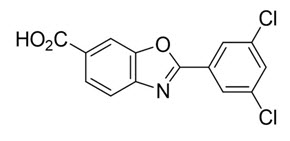
The chemical name of tafamidis is 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid. The molecular formula is C14H7Cl2NO3, and the molecular weight is 308.12 g/mol. The structural formula is:
Tafamidis 61-mg soft gelatin capsule for oral use contains a white to pink colored suspension of tafamidis 61 mg and the following inactive ingredients: ammonium hydroxide 28%, butylated hydroxytoluene, gelatin, glycerin, iron oxide (red), polyethylene glycol 400, polysorbate 20, povidone (K-value 90), polyvinyl acetate phthalate, propylene glycol, sorbitol, and titanium dioxide.
Mechanism of Action
Tafamidis is a selective stabilizer of TTR. Tafamidis binds to TTR at the thyroxine binding sites, stabilizing the tetramer and slowing dissociation into monomers, the rate-limiting step in the amyloidogenic process.
Before taking tafamidis, tell your doctor:
- If you are allergic to this medicine (tafamidis); any part of this medicine (tafamidis); or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you are breast-feeding. Do not breast-feed while you take this medicine (tafamidis).
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this medicine (tafamidis) with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take tafamidis?
- Tell all of your health care providers that you take this medicine (tafamidis). This includes your doctors, nurses, pharmacists, and dentists.
- You may need to use birth control to prevent pregnancy while taking this medicine (tafamidis). Talk with your doctor.
- This medicine may cause harm to the unborn baby if you take it while you are pregnant. If you are pregnant or you get pregnant while taking this medicine (tafamidis), call your doctor right away.
How is tafamidis best taken?
Use this medicine (tafamidis) as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Swallow whole. Do not chew, break, or crush.
- Keep taking this medicine (tafamidis) as you have been told by your doctor or other health care provider, even if you feel well.
What do I do if I miss a dose?
- Take a missed dose as soon as you think about it.
- If it is close to the time for your next dose, skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of tafamidis that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
What are some other side effects of tafamidis?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out tafamidis?
- Store at room temperature.
- Store in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
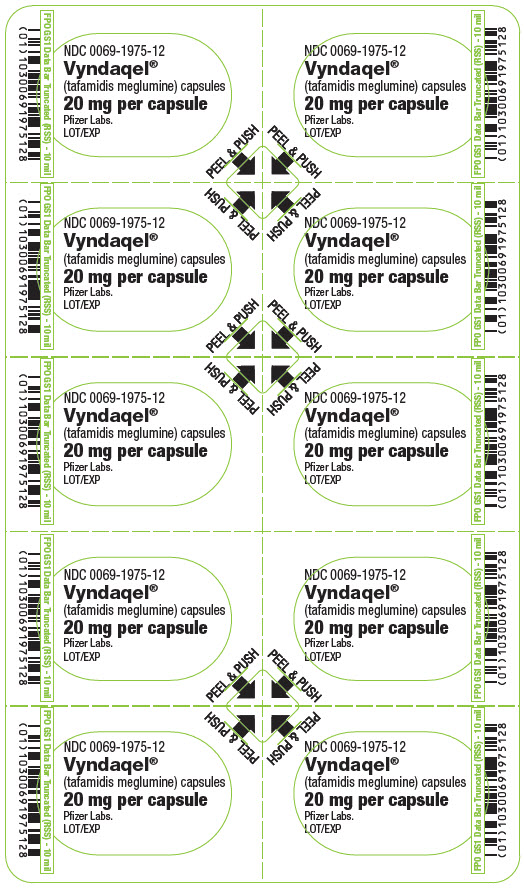
PRINCIPAL DISPLAY PANEL – 20 MG CAPSULE BLISTER CARD
- NDC 0069-1975-12
- Vyndaqel®
(tafamidis meglumine) capsules - 20 mg per capsule
- Pfizer Labs.
LOT/EXP - PEEL & PUSH
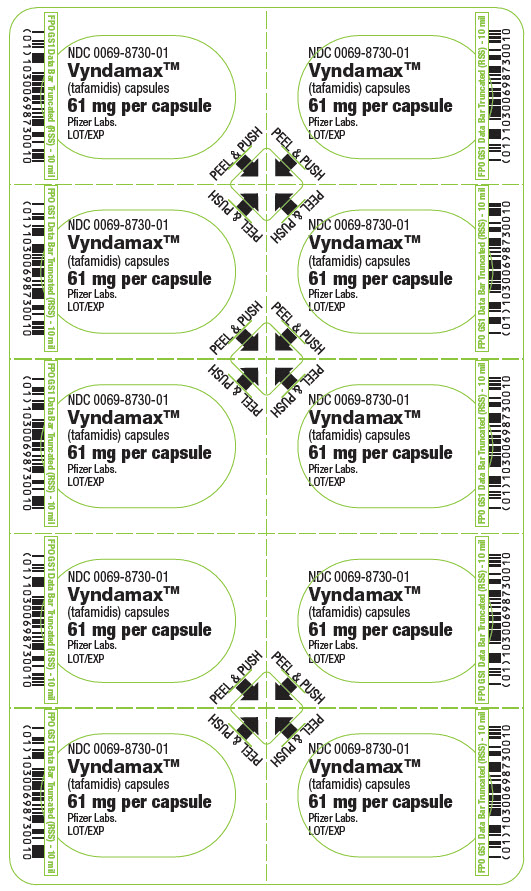
PRINCIPAL DISPLAY PANEL – 61 MG CAPSULE BLISTER CARD
- NDC 0069-8730-01
- Vyndamax™
(tafamidis) capsules - 61 mg per capsule
- Pfizer Labs.
LOT/EXP - PEEL & PUSH
SRC: NLM .