Tivdak
Generic name: tisotumab vedotin-tftv
Dosage form: lyophilized powder for injection, for intravenous use
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Tivdak?
Tivdak is a prescription medicine used to treat adults with cervical cancer, that has returned or has spread to other parts of the body, and who have received chemotherapy that did not work or is no longer working.
It is not known if Tivdak is safe and effective in children.
Description
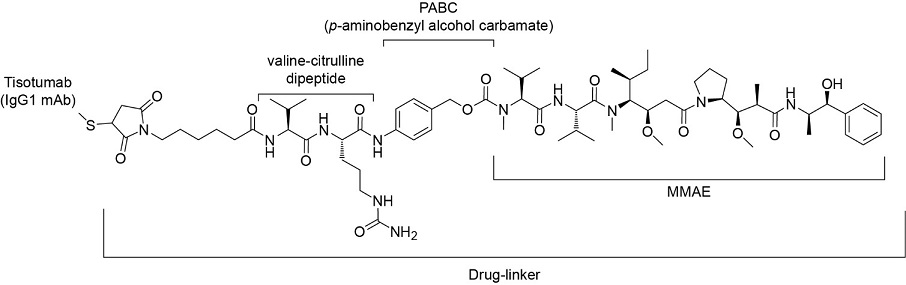
Tisotumab vedotin-tftv is a Tissue Factor (TF) directed antibody drug conjugate (ADC) comprised of a human anti-TF IgG1-kappa antibody conjugated to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease-cleavable vc (valine-citrulline) linker. The monoclonal antibody is produced in a mammalian cell cline (Chinese hamster ovary). MMAE and the linker are produced by chemical synthesis. Each monoclonal antibody molecule carries an average of 4 MMAE molecules. Tisotumab vedotin-tftv has an approximate molecular weight of 153 kDa. The chemical structure is as follows:
. Structural Formula
TIVDAK (tisotumab vedotin-tftv) for injection, is provided as a sterile, preservative-free, white to off-white lyophilized cake or powder in a single-dose vial for infusion after dilution. Following reconstitution with 4 mL of Sterile Water for Injection, a clear to slightly opalescent, colorless to brownish-yellow solution containing 10 mg/mL tisotumab vedotin-tftv is produced [see Dosage and Administration . Each mL of reconstituted solution contains 10 mg of tisotumab vedotin-tftv, d-mannitol (30 mg), l-histidine (2.11 mg), l-histidine monohydrochloride (3.44 mg), and sucrose (30 mg), at pH 6.0.
Mechanism of Action
Tisotumab vedotin-tftv is a tissue factor (TF)-directed antibody drug conjugate (ADC). The antibody is a human IgG1 directed against cell surface TF. TF is the primary initiator of the extrinsic blood coagulation cascade. The small molecule, MMAE, is a microtubule-disrupting agent, attached to the antibody via a protease-cleavable linker. Nonclinical data suggests that the anticancer activity of tisotumab vedotin-tftv is due to the binding of the ADC to TF expressing cancer cells, followed by internalization of the ADC-TF complex, and release of MMAE via proteolytic cleavage. MMAE disrupts the microtubule network of actively dividing cells, leading to cell cycle arrest and apoptotic cell death. In vitro, tisotumab vedotin-tftv also mediates antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity.
What is the most important information I should know about Tivdak?
Tivdak can cause serious side effects, including:
- Eye problems. Eye problems are common with Tivdak, and can also be serious. Tivdak can cause changes to the surface of your eye that can lead to dry eyes, eye redness, eye irritation, corneal ulcers, blurred vision, and severe vision loss. Tell your healthcare provider if you develop new or worsening vision changes or eye problems during treatment with Tivdak.
- Your healthcare provider will send you to an eye specialist to check your eyes before you start treatment with Tivdak, before each dose of Tivdak, and as needed for any new or worsening signs and symptoms of eye problems.
- Your healthcare provider will prescribe 3 different types of eye drops before you start treatment with Tivdak. Bring the eye drops with you to each infusion and use them as directed by your healthcare provider to reduce your risk of eye problems:
- You should use steroid eye drops before each infusion and as prescribed for 72 hours after each infusion.
- You should use vasoconstrictor eye drops right before each infusion.
- You should use lubricating eye drops throughout treatment and for 30 days after your last dose of Tivdak.
- Do not wear contact lenses throughout your treatment with Tivdak unless you are told to use them by your eye specialist.
See “What are the possible side effects of Tivdak?” for more information about side effects.
What should I tell my healthcare provider before using Tivdak?
Before receiving Tivdak, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of vision or eye problems
- have numbness or tingling in your hands or feet
- have bleeding problems
- have liver problems
- are pregnant or plan to become pregnant. Tivdak can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Tivdak.
- Females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with Tivdak.
- You should use an effective birth control during treatment and for 2 months after your last dose of Tivdak.
- Males with female partners who are able to become pregnant:
- You should use an effective birth control during treatment and for 4 months after your last dose of Tivdak.
- Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if Tivdak passes into your breast milk. Do not breastfeed during treatment and for 3 weeks after your last dose of Tivdak.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Tivdak with certain other medicines may cause side effects.
How should I use Tivdak?
- Tivdak will be given to you by intravenous (IV) infusion into your vein over 30 minutes.
- Tivdak is usually given every 3 weeks.
- Your healthcare provider will decide how many infusions you need.
- Your healthcare provider will put cold packs on your eyes during each infusion. Your healthcare provider may decrease your dose, temporarily stop, or completely stop treatment with Tivdak if you have side effects.
What are the possible side effects of Tivdak?
Tivdak can cause serious side effects, including:
- See “What is the most important information I should know about Tivdak?”
- Peripheral neuropathy. Nerve problems called peripheral neuropathy are common with Tivdak, and can also be serious. Tell your healthcare provider right away if you get numbness or tingling in your hands or feet or muscle weakness.
- Bleeding (hemorrhage). Bleeding problems are common with Tivdak, and can also be serious. Tell your healthcare provider right away if you get signs or symptoms of bleeding during treatment with Tivdak, including:
- blood in your stools or black stools (looks like tar)
- blood in your urine
- cough up or vomit blood
- unusual vaginal bleeding
- any unusual or heavy bleeding
- Lung problems. Tivdak may cause severe or life-threatening inflammation of the lungs that can lead to death. Tell your healthcare provider right away if you get new or worsening symptoms, including trouble breathing, shortness of breath, or cough.
The most common side effects of Tivdak include:
- decreased red blood cell and white blood cell counts
- tiredness
- nausea
- hair loss (alopecia)
- nosebleed
- changes in kidney function blood tests
- dry eye
- abnormal blood clotting test results
- diarrhea
- rash
Tivdak may cause fertility problems in males, which may affect your ability to father children. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of Tivdak.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tivdak
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about Tivdak, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Tivdak that is written for healthcare professionals.
What are the ingredients in Tivdak?
Active ingredient: tisotumab vedotin-tftv
Inactive ingredients: d-mannitol, l-histidine, l-histidine monohydrochloride, and sucrose.
How Supplied
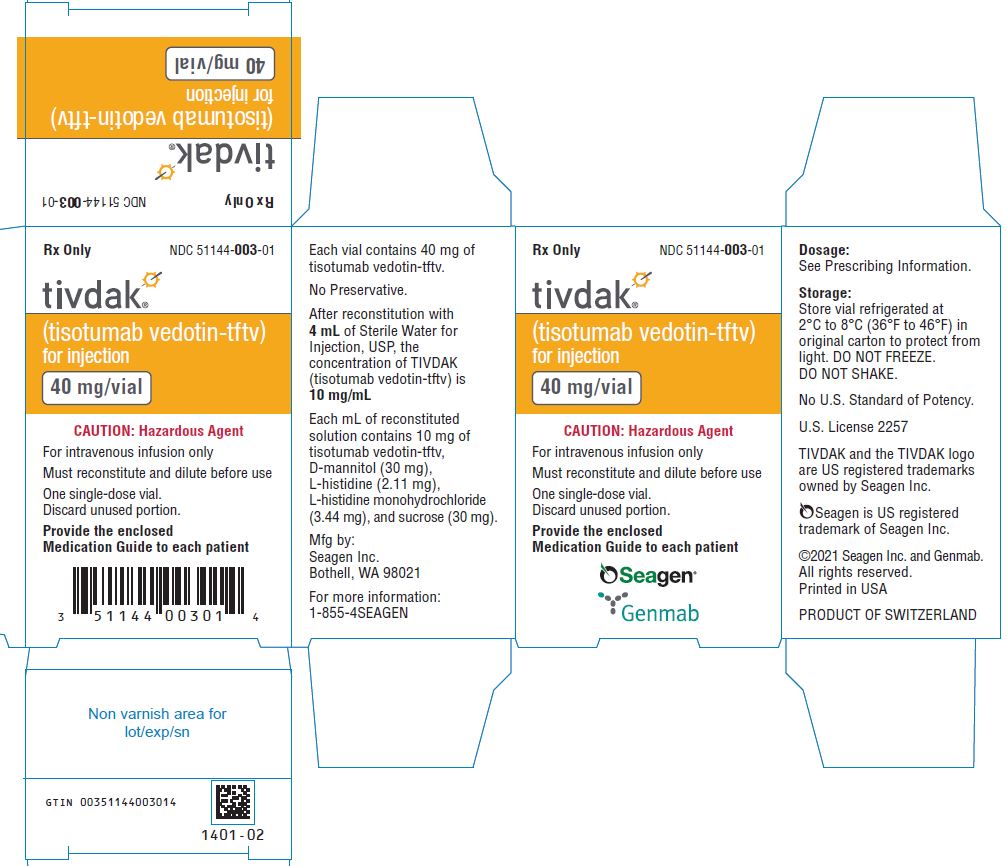
TIVDAK (tisotumab vedotin-tftv) is supplied as a white to off-white lyophilized cake or powder in a 40 mg single-dose vial for reconstitution. TIVDAK vials are available in the following packages:
- Carton of one 40 mg single-dose vial [NDC 51144-003-01]
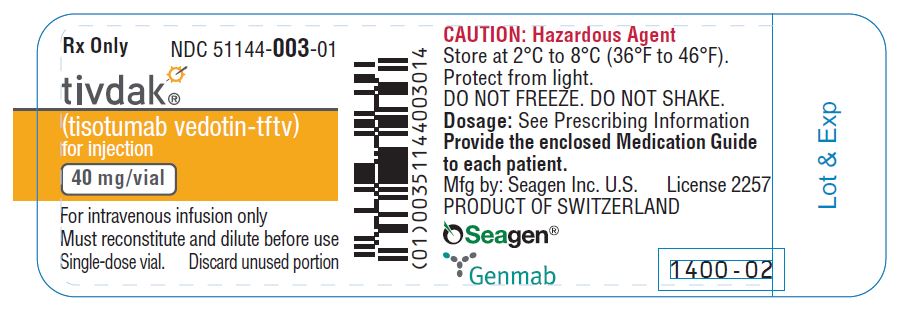
Storage
Store TIVDAK vials refrigerated at 2ºC to 8ºC (36ºF to 46ºF) in the original carton to protect from light. Do not freeze. Do not shake.
Label
PRINCIPAL DISPLAY PANEL
- (tisotumab vedotin-tftv)
for injection - 40 mg/vial
- CAUTION: Hazardous Agent
- For intravenous infusion only
- Must reconstitute and dilute before use
- One single-dose vial.
Discard unused portion. - Provide the enclosed
Medication Guide to each patient