Testim
Generic name: testosterone topical
Brand names: Androderm, AndroGel Packets, AndroGel Pump 1.25 g/actuation, FIRST-Testosterone, Fortesta, Testim, Vogelxo
Drug class: Androgens and anabolic steroids
Medically reviewed by A Ras MD.
What is Testim?
Testim is a prescription medicine that contains testosterone. Testim is used to treat adult males who have low or no testosterone due to certain medical conditions.
- Your healthcare provider will test your blood before you start and while you are using Testim.
- It is not known if Testim is safe or effective to treat men who have low testosterone due to aging.
- It is not known if Testim is safe or effective in children younger than 18 years old. Improper use of testosterone in children may affect bone growth.
Testim is a controlled substance (CIII) because it contains testosterone that can be a target for people who abuse prescription medicines. Keep your Testim in a safe place to protect it. Never give your Testim to anyone else, even if they have the same symptoms you have. Selling or giving away this medicine may harm others and it is against the law.
Testim is not meant for use in women.
Description
TESTIM (testosterone gel) is a clear to translucent hydroalcoholic topical gel containing testosterone, an androgen. TESTIM provides continuous transdermal delivery of testosterone for 24 hours, following a single application to intact, clean, dry skin of the shoulders and/or upper arms.
One 5-g or two 5-g tubes of TESTIM contains 50 mg or 100 mg of testosterone, respectively, to be applied daily to the skin’s surface. Approximately 10% of the applied testosterone dose is absorbed across skin of average permeability during a 24-hour period.
The active pharmacological ingredient in TESTIM is testosterone. Testosterone USP is a white to practically white crystalline powder chemically described as 17-β hydroxyandrost-4-en-3-one. The structural formula is shown in the following figure:
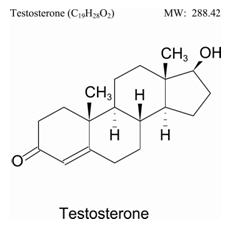
Testim may have an alcoholic/musk odor. Inactive ingredients in TESTIM are purified water, pentadecalactone, carbopol, acrylates, propylene glycol, glycerin, polyethylene glycol, ethanol (74%), and tromethamine.
Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as facial, pubic, chest and axillary hair; laryngeal enlargement; vocal cord thickening; and alterations in body musculature and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has 2 main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter’s syndrome or Leydig cell aplasia, while secondary hypogonadism (hypogonadotropic hypogonadism) is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).
What is the most important information I should know about Testim?
1. Testim can transfer from your body to others including, children and women. Children and women should avoid contact with the unwashed or not covered (unclothed) areas where Testim has been applied to your skin. Early signs and symptoms of puberty have occurred in young children who have come in direct contact with testosterone by touching areas where men have used Testim.
Children
Signs and symptoms of early puberty in a child when they come in direct contact with Testim may include:
Abnormal sexual changes:
- enlarged penis or clitoris.
- early growth of hair near the vagina or around the penis (pubic hair).
- erections or acting out sexual urges (sex drive).
Behavior problems:
- acting aggressively, behaving in an angry or violent way.
Women
Signs and symptoms in women when they come in direct contact with Testim may include:
- changes in body hair.
- an abnormal increase in pimples (acne).
Stop using Testim and call your healthcare provider right away if you see any signs and symptoms in a child or a woman that may have happened through accidental touching of the area where you have placed Testim.
2. To lower the risk of transfer of Testim from your body to others, follow these important instructions:
- Apply Testim only to the areas of your shoulders and upper arms that will be covered by a short sleeve t-shirt.
- Wash your hands right away with soap and water after applying Testim.
- After the gel has dried, cover the application area with clothing. Keep the area covered until you have washed the application area well or have showered.
- If you expect to have skin-to-skin contact with another person, first wash the application area well with soap and water.
- If a child or woman touches the area where you have applied Testim, that area on the child or woman should be washed well with soap and water right away.
Who should not use Testim?
Do not use Testim if you:
- have breast cancer.
- have or might have prostate cancer.
- are pregnant or may become pregnant or are breastfeeding. Testim may harm your unborn or breastfeeding baby.
- Women who are pregnant or who may become pregnant should avoid contact with the area of skin where Testim has been applied.
What should I tell my healthcare provider before using Testim?
Before using Testim, tell your healthcare provider about all of your medical conditions including if you:
- have breast cancer.
- have or might have prostate cancer.
- have urinary problems due to an enlarged prostate.
- have heart problems.
- have liver or kidney problems.
- have problems breathing while you sleep (sleep apnea).
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using Testim with certain other medicines can affect each other. Especially, tell your healthcare provider if you take:
- insulin
- medicines that decrease blood clotting (blood thinners)
- corticosteroids
How should I use Testim?
- See the detailed Instructions for Use that come with Testim for information about how to use Testim.
- It is important that you apply Testim exactly as your healthcare provider tells you to.
- Your healthcare provider may change your Testim dose. Do not change your Testim dose without talking to your healthcare provider.
- Apply Testim at the same time each morning. Testim should be applied after showering or bathing.
What are the possible side effects of Testim?
Testim can cause serious side effects including:
See “What is the most important information I should know about Testim?”
- If you already have enlargement of your prostate gland your signs and symptoms can get worse while using Testim. This can include:
- increased urination at night.
- trouble starting your urine stream.
- having to pass urine many times during the day.
- having an urge to go to the bathroom right away.
- having a urine accident.
- being unable to pass urine or weak urine flow.
- Possible increased risk of prostate cancer. Your healthcare provider should check you for prostate cancer or any other prostate problems before you start and while you use Testim.
- Blood clots in the legs or lungs. Signs and symptoms of a blood clot in your leg can include leg pain, swelling or redness. Signs and symptoms of a blood clot in your lungs can include difficulty breathing or chest pain.
- Possible increased risk of heart attack or stroke.
- In large doses Testim may lower your sperm count.
- Swelling of your ankles, feet, or body, with or without heart failure. This may cause serious problems for people who have heart, kidney or liver disease.
- Enlarged or painful breasts.
- Having problems breathing while you sleep (sleep apnea).
Call your healthcare provider right away if you have any of the serious side effects listed above.
The most common side effects of Testim include:
- skin irritation where Testim is applied
- increased red blood cell count
- headache
- increased blood pressure
Other side effects include more erections than are normal for you or erections that last a long time.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Testim. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Testim
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Testim for a condition for which it was not prescribed. Do not give Testim to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about Testim that is written for health professionals.
How should I store Testim?
- Store Testim between 59ºF to 86ºF (15ºC to 30ºC).
- Safely throw away used Testim in household trash. Be careful to prevent accidental exposure of children or pets.
- Keep Testim away from fire.
Keep Testim and all medicines out of the reach of children.
What are the ingredients in Testim?
Active ingredient: testosterone
Inactive ingredients: purified water, pentadecalactone, carbopol, acrylates, propylene glycol, glycerin, polyethylene glycol, ethanol (74%), and tromethamine.
Label
Package Label – Principal Display Panel – 5 g Tube Label – NDC 66887-001-01

Package Label – Principal Display Panel – 30 Unit-dose Tubes Carton – NDC 66887-001-05
