Toviaz
Generic name: fesoterodine
Drug class: Urinary antispasmodics
Medically reviewed by A Ras MD
What is Toviaz?
Toviaz is a prescription medicine used in adults to treat symptoms of a condition called overactive bladder, including:
- Urge urinary incontinence — leaking or wetting accidents due to a strong need to urinate,
- Urinary urgency — having a strong need to urinate right away,
- Urinary frequency — having to urinate too often.
Toviaz has not been studied in children.
Description
Toviaz contains fesoterodine fumarate and is an extended-release tablet. Fesoterodine is rapidly de-esterified to its active metabolite (R)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethyl-phenol, or 5-hydroxymethyl tolterodine, which is a muscarinic receptor antagonist.
Chemically, fesoterodine fumarate is designated as isobutyric acid 2-((R)-3-diisopropylammonium-1-phenylpropyl)-4-(hydroxymethyl) phenyl ester hydrogen fumarate. The empirical formula is C30H41NO7 and its molecular weight is 527.66. The structural formula is:
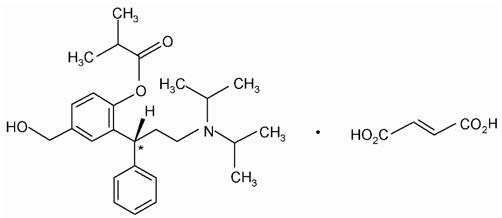
The asterisk (*) indicates the chiral carbon.
Fesoterodine fumarate is a white to off-white powder, which is freely soluble in water. Each Toviaz extended-release tablet contains either 4 mg or 8 mg of fesoterodine fumarate and the following inactive ingredients: glyceryl behenate, hypromellose, indigo carmine aluminum lake, lactose monohydrate, soya lecithin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and xylitol.
Mechanism of Action
Fesoterodine is a competitive muscarinic receptor antagonist. After oral administration, fesoterodine is rapidly and extensively hydrolyzed by nonspecific esterases to its active metabolite, 5-hydroxymethyl tolterodine, which is responsible for the antimuscarinic activity of fesoterodine.
Muscarinic receptors play a role in contractions of urinary bladder smooth muscle. Inhibition of these receptors in the bladder is presumed to be the mechanism by which fesoterodine produces its effects.
Who should not take Toviaz?
- Are not able to empty your bladder (urinary retention)
- Have delayed or slow emptying of your stomach (gastric retention)
- Have an eye problem called “uncontrolled narrow-angle glaucoma”
- Are allergic to Toviaz or any of its ingredients. See the end of this guide for a complete list of ingredients.
- Are allergic to Detrol or Detrol LA, which contains tolterodine.
What should I tell my healthcare provider before taking Toviaz?
Before starting Toviaz, tell your doctor about all of your medical and other conditions that may affect the use of Toviaz, including:
- Stomach or intestinal problems or problems with constipation
- Problems emptying your bladder or if you have a weak urine stream
- Treatment for an eye problem called narrow-angle glaucoma
- Kidney problems
- Liver problems
- A condition called myasthenia gravis
- If you are pregnant or trying to become pregnant. It is not known if Toviaz can harm your unborn baby.
- If you are breastfeeding. It is not known if Toviaz passes into breast milk or if it can harm your baby. Talk to your doctor about the best way to feed your baby if you take Toviaz.
Before starting on Toviaz, tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal products. Toviaz may affect the way other medicines work, and other medicines may affect how Toviaz works. Especially tell your doctor if you are taking antibiotics or antifungal medicines.
Know all the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I take Toviaz?
- Take Toviaz exactly as your doctor tells you to take it.
- Your doctor may give you the lower 4 mg dose of Toviaz if you have certain medical conditions, such as severe kidney problems.
- Take Toviaz with liquid and swallow the tablet whole. Do not chew, divide, or crush the tablet.
- You can take Toviaz with or without food.
- If you miss a dose of Toviaz, begin taking Toviaz again the next day. Do not take 2 doses of Toviaz in the same day.
If you take too much Toviaz, call your doctor or go to an emergency department right away.
What should I avoid while taking Toviaz?
What else should I keep in mind while taking Toviaz?
- Do not drive, operate machinery, or do other dangerous activities until you know how Toviaz affects you. Blurred vision, dizziness, and drowsiness are possible side effects of medicines such as Toviaz.
- Use caution in hot environments. Decreased sweating and severe heat illness can occur when medicines such as Toviaz are used in a hot environment.
- Drinking alcohol while taking medicines such as Toviaz may cause increased drowsiness.
Label
PRINCIPAL DISPLAY PANEL – 4 MG TABLET BOTTLE LABEL
- NDC 0069-0242-30
- 30 Tablets
Rx only - Toviaz®
(fesoterodine fumarate)
extended release tablets - 4 mg
- Pfizer
Distributed by
Pfizer Labs
Division of Pfizer Inc, NY, NY 10017


PRINCIPAL DISPLAY PANEL – 8 MG TABLET BOTTLE LABEL
- NDC 0069-0244-30
- 30 Tablets
Rx only - Toviaz®
(fesoterodine fumarate)
extended release tablets - 8 mg
- Pfizer
Distributed by
Pfizer Labs
Division of Pfizer Inc, NY, NY 10017

What are the possible side effects of Toviaz?
Toviaz may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat or tongue. If you experience these symptoms, you should stop taking Toviaz and get emergency medical help right away.
The most common side effects of Toviaz are:
- Dry mouth
- Constipation
Toviaz may cause other less common side effects, including:
- Dry eyes
- Trouble emptying the bladder
Tell your doctor if you have any side effects that bother you or that do not go away.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
These are not all of the possible side effects of Toviaz. For a complete list, ask your doctor.
General information about the safe and effective use of Tovia
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Only use Toviaz the way your doctor tells you. Do not give Toviaz to other people, even if they have the same symptoms you have. It may harm them.
This guide summarizes the most important information about Toviaz. If you would like more information, talk with your doctor. You can ask your doctor for information about Toviaz that is written for healthcare professionals. You can also call 1-877-9-Toviaz (1-877-986-8429) or go to www.Toviaz.com.
How should I store Toviaz?
- Store Toviaz at room temperature, 68° to 77°F (20° to 25°C); brief periods permitted between 59° to 86°F (15° to 30°C)
- Protect the medicine from moisture by keeping the bottle closed tightly.
- Safely throw away Toviaz that is out of date or no longer needed.
Keep Toviaz and all medicines out of the reach of children.
What are the ingredients in Tovia?
Active ingredient: fesoterodine fumarate
Inactive ingredients: glyceryl behenate, hypromellose, indigo carmine aluminum lake, lactose monohydrate, soya lecithin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and xylitol.
SRC: NLM .