OctreoScan
Generic name: Indium In-111 Pentetreotide
Drug class: Diagnostic radiopharmaceuticals
Medically reviewed by A Ras MD.
What is OctreoScan used for?
OctreoScan is used as a test to check for tumors.
Description
Octreoscan™ is a kit for the preparation of Indium In 111 Pentetreotide Injection, a radioactive diagnostic agent. It is a kit consisting of two components:
1) A 10-mL Octreoscan Reaction Vial which contains a lyophilized mixture of:
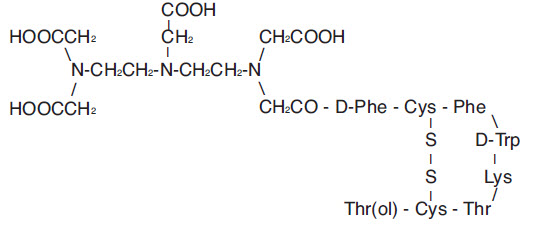
(i) 10 mcg pentetreotide [N-(diethylenetriamine-N,N,N′,N″-tetraacetic acid-N″-acetyl)-D-phenylalanyl-L-hemicystyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-L-hemicystyl-L-threoninol cyclic (2→7) disulfide], (also known as octreotide DTPA),
(ii) 2 mg gentisic acid [2, 5-dihydroxybenzoic acid],
(iii) 4.9 mg trisodium citrate, anhydrous,
(iv) 0.37 mg citric acid, anhydrous, and
(v) 10 mg inositol.
Pentetreotide has the following structural formula:
 Prior to lyophilization, sodium hydroxide or hydrochloric acid may have been added for pH adjustment. The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
Prior to lyophilization, sodium hydroxide or hydrochloric acid may have been added for pH adjustment. The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
2) A 10-mL vial of Indium In 111 Chloride Solution, which contains: 1.1 mL or 111 MBq/mL (3 mCi/mL) indium In 111 chloride in 0.02N HCl at time of calibration. The vial also contains ferric chloride at a concentration of 3.5 mcg/mL (ferric ion, 1.2 mcg/mL). The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
Indium In 111 Pentetreotide Injection is prepared by combining the two kit components . Indium In-111 reacts with the diethylenetriaminetetraacetic acid portion of the pentetreotide molecule to form indium In 111 pentetreotide. The pH of the resultant Indium In 111 Pentetreotide Injection is between 3.8 and 4.3. No bacteriostatic preservative is present.
The Indium In 111 Pentetreotide Injection is suitable for intravenous administration as is, or it may be diluted to a maximum volume of 3 mL with 0.9% Sodium Chloride Injection, USP, immediately before intravenous administration. In either case, the radiolabeling yield of Indium In 111 Pentetreotide Injection should be determined before administration to the patient. A method recommended for determining the radiolabeling yield is presented at the end of this package insert.
Before taking OctreoScan, tell your doctor:
- If you are allergic to OctreoScan; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take OctreoScan with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take OctreoScan?
- Tell all of your health care providers that you take OctreoScan. This includes your doctors, nurses, pharmacists, and dentists.
- This medicine is radioactive. You will need to follow what the doctor has told you to lessen being exposed to OctreoScan.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is OctreoScan best taken?
Use OctreoScan as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into a vein.
- Your doctor may tell you to use a laxative like bisacodyl or lactulose before and after getting OctreoScan. Follow what your doctor has told you.
- Drink lots of noncaffeine liquids before, during, and after using OctreoScan unless you are told to drink less liquid by your doctor.
- You will need to empty your bladder often after the test is over as your doctor has told you.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of OctreoScan that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of low blood sugar like dizziness, headache, feeling sleepy, feeling weak, shaking, a fast heartbeat, confusion, hunger, or sweating.
What are some other side effects of OctreoScan?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if you have any side effects that bother you or do not go away.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out OctreoScan?
- If you need to store OctreoScan at home, talk with your doctor, nurse, or pharmacist about how to store it.
Label
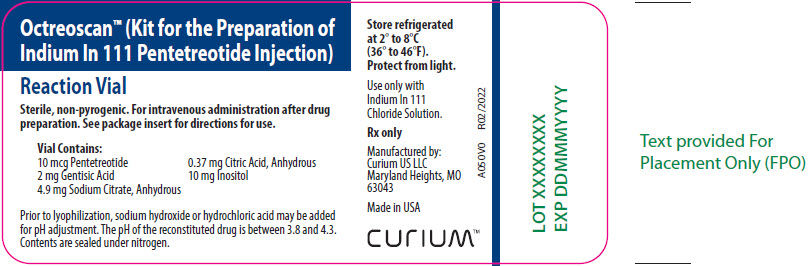
PRINCIPAL DISPLAY PANEL – A050V0
- Octreoscan™ (Kit for the Preparation of Indium In 111 Pentetreotide Injection)
- Reaction Vial
- Sterile, non-pyrogenic. For intravenous administration after drug preparation. See package insert for directions for use.
- Vial Contains:
10 mcg Pentetreotide
2 mg Gentisic Acid
4.9 mg Sodium Citrate, Anhydrous
0.37 mg Citric Acid, Anhydrous
10 mg Inositol - Prior to lyophilization, sodium hydroxide or hydrochloric acid may be added for pH adjustment. The pH of the reconstituted drug is between 3.8 and 4.3. Contents are sealed under nitrogen.
- Store refrigerated at 2° to 8°C (36° to 46°F). Protect from light.
- Use only with Indium In 111 Chloride Solution.
- Rx only
- Manufactured by:
Curium US LLC
Maryland Heights, MO 63043 - Made in USA
- CURIUM™
- A050V0
- R02/2022
- LOT XXXXXXXX
- EXP DDMMMYYYY
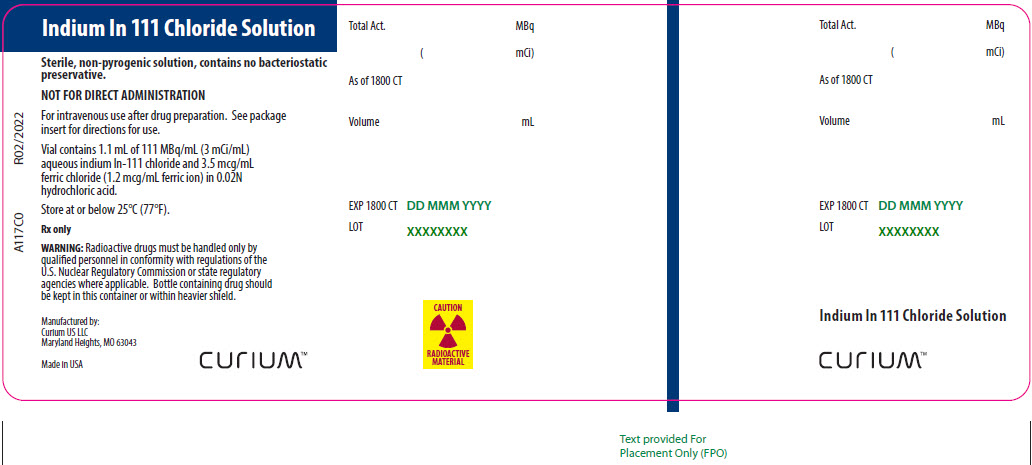
PRINCIPAL DISPLAY PANEL – A117C0
- Indium In 111 Chloride Solution
- Sterile, non-pyrogenic solution, contains no bacteriostatic preservative.
- NOT FOR DIRECT ADMINISTRATION
- For intravenous use after drug preparation. See package insert for directions for use.
- Vial contains 1.1 mL of 111 MBq/mL (3 mCi/mL) aqueous indium In-111 chloride and 3.5 mcg/mL ferric chloride (1.2 mcg/mL ferric ion) in 0.02N hydrochloric acid.
- Store at or below 25°C (77°F).
- Rx only
- WARNING: Radioactive drugs must be handled only by qualified personnel in conformity with regulations of the U.S. Nuclear Regulatory Commission or state regulatory agencies where applicable. Bottle containing drug should be kept in this container or within heavier shield.
- Manufactured by:
Curium US LLC
Maryland Heights, MO 63043 - Made in USA
- CURIUM™
- CAUTION RADIOACTIVE MATERIAL
- A117C0
- R02/2022
- Total Act. MBq
- ( mCi)
- As of 1800 CT
- Volume mL
- EXP 1800 CT DD MMM YYYY
- LOT XXXXXXXX
SRC: NLM .