Oriahnn
Generic name: elagolix, estradiol, and norethindrone
Drug class: Sex hormone combinations
Medically reviewed by A Ras MD.
What is Oriahnn?
Oriahnn is a prescription medicine used to control heavy menstrual bleeding in premenopausal women (before “change of life” or menopause) with uterine fibroids.
It is not known if Oriahnn is safe and effective in children under 18 years of age.
Description
ORIAHNN consists of two capsules: one to be taken orally in the morning (AM) and one to be taken orally in the evening (PM). The AM capsule is white and yellow and contains 300 mg elagolix (equivalent to 310.4 mg of elagolix sodium), 1 mg estradiol, and 0.5 mg norethindrone acetate. The PM capsule is white and light blue and contains 300 mg of elagolix (equivalent to 310 mg of elagolix sodium).
Elagolix
Elagolix sodium is the sodium salt of the active moiety elagolix, a nonpeptide small molecule, GnRH receptor antagonist. Elagolix sodium is chemically described as sodium 4-({(1R)-2-[5-(2-fluoro-3-methoxyphenyl)-3-{[2-fluoro-6-(trifluoromethyl)phenyl]methyl}-4-methyl-2,6-dioxo-3,6-dihydropyrimidin-1(2H)-yl]-1-phenylethyl}amino)butanoate. Elagolix sodium has a molecular formula of C32H29F5N3O5Na and a molecular weight of 653.58. Elagolix free acid has a molecular formula of C32H30F5N3O5 and a molecular weight of 631.60.
Elagolix sodium has the following structural formula:
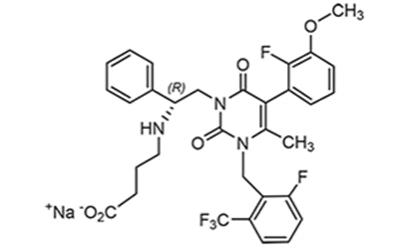
Elagolix sodium is a white to off-white to light yellow powder and is freely soluble in water.
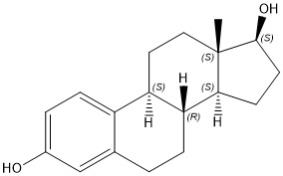
Estradiol
Estradiol (E2), an estrogen, is a white or almost white crystalline powder. Its chemical name is estra-1,3,5(10)-triene-3,17β-diol with the molecular formula of C18H24O2, and molecular weight of 272.38. The structural formula of E2 is as follows:
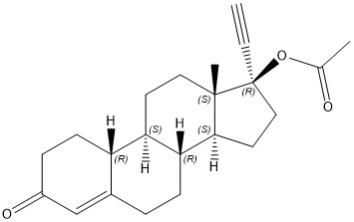
Norethindrone acetate
Norethindrone acetate (NETA), a progestin, is a white or yellowish white crystalline powder. Its chemical name is 17β-acetoxy-19-nor-17α-pregn-4-en-20-yn-3-one with the molecular formula of C22H28O3 and molecular weight of 340.46.
ORIAHNN morning (AM) capsules contain the following inactive ingredients: anhydrous sodium carbonate, polyethylene glycol 3350, crospovidone, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, purified water, lactose monohydrate, starch (corn), copovidone, talc, hypromellose, triacetin, and gelatin capsule shell. The capsule shell contains the following ingredients: FD&C Red #40, FD&C Yellow #5 [see Warnings and Precautions (5.12)], FD&C Yellow #6, titanium dioxide, gelatin, and printing ink (shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water).
ORIAHNN evening (PM) capsules contain the following inactive ingredients: anhydrous sodium carbonate, polyethylene glycol 3350, crospovidone, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, purified water, and gelatin capsule shell. The capsule shell contains the following ingredients: FD&C Blue #2, FDA/E172 yellow iron oxide, titanium dioxide, gelatin, and printing ink (shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water).
Mechanism of Action
ORIAHNN combines elagolix and estradiol/norethindrone acetate (E2/NETA), a combination of estrogen and progestin.
Elagolix is a GnRH receptor antagonist that inhibits endogenous GnRH signaling by binding competitively to GnRH receptors in the pituitary gland. Administration of elagolix results in dose-dependent suppression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to decreased blood concentrations of the ovarian sex hormones estradiol and progesterone and reduces bleeding associated with uterine fibroids.
E2 acts by binding to nuclear receptors that are expressed in estrogen-responsive tissues. As a component of ORIAHNN, the addition of exogenous estradiol may reduce the increase in bone resorption and resultant bone loss that can occur due to a decrease in circulating estrogen from elagolix alone.
Progestins such as NETA act by binding to nuclear receptors that are expressed in progesterone-responsive tissues. As a component of ORIAHNN, NETA may protect the uterus from the potential adverse endometrial effects of unopposed estrogen.
What is the most important information I should know about Oriahnn?
Oriahnn may cause serious side effects, including:
- cardiovascular conditions
- Oriahnn may increase your chances of heart attack, stroke, or blood clots, especially if you are over 35 years of age and smoke, have uncontrolled high blood pressure, high cholesterol, diabetes, or are obese. Stop taking Oriahnn and call your healthcare provider right away or go to the nearest hospital emergency room right away if you have:
- leg pain or swelling that will not go away
- sudden shortness of breath
- double vision, bulging of the eyes, sudden blindness, partial or complete
- pain or pressure in your chest, arm, or jaw
- sudden, severe headache unlike your usual headaches
- weakness or numbness in an arm or leg, or trouble speaking
- Oriahnn may increase your chances of heart attack, stroke, or blood clots, especially if you are over 35 years of age and smoke, have uncontrolled high blood pressure, high cholesterol, diabetes, or are obese. Stop taking Oriahnn and call your healthcare provider right away or go to the nearest hospital emergency room right away if you have:
- bone loss (decreased bone mineral density)
- While you are taking Oriahnn, your estrogen levels may be low. Low estrogen levels can lead to bone mineral density loss.
- If you have bone loss on Oriahnn, your bone density may improve after you stop taking Oriahnn, but complete recovery may not occur. It is unknown if these bone changes could increase your risk for broken bones as you age. For this reason, you should not take Oriahnn for more than 24 months.
- Your healthcare provider may order an X-ray test called a DXA scan to check your bone mineral density when you start taking Oriahnn and periodically after you start.
- Your healthcare provider may advise you to take vitamin D and calcium supplements as part of a healthy lifestyle that promotes bone health. Iron supplements should not be taken at the same time that you take vitamin D and calcium supplements.
- effects on pregnancy
- Do not take Oriahnn if you are trying to become pregnant or are pregnant. It may increase the risk of early pregnancy loss.
- If you think you may be pregnant, stop taking Oriahnn right away and call your healthcare provider.
- If you become pregnant while taking Oriahnn, you are encouraged to enroll in the Pregnancy Registry. The purpose of the pregnancy registry is to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-833-782-7241.
- Oriahnn can decrease your menstrual bleeding or result in no menstrual bleeding at all, making it hard to know if you are pregnant. Watch for other signs of pregnancy such as breast tenderness, weight gain, and nausea.
- Oriahnn does not prevent pregnancy. You will need to use effective methods of birth control while taking Oriahnn and for 1 week after you stop taking Oriahnn. Examples of effective methods can include condoms or spermicide, which do not contain hormones.
- Talk to your healthcare provider about which birth control to use during treatment with Oriahnn. Your healthcare provider may change the birth control you were on before you start taking Oriahnn.
Who should not take Oriahnn?
Do not take Oriahnn if you:
- have or have had:
- stroke or a heart attack
- a problem that makes your blood clot more than normal
- blood circulation disorder
- certain heart valve problems or heart rhythm abnormalities that can cause blood clots to form in the heart
- blood clots in your legs (deep vein thrombosis), lungs (pulmonary embolism), or eyes (retinal thrombosis)
- high blood pressure not well controlled by medicine
- diabetes with kidney, eye, nerve, or blood vessel damage
- certain kinds of headaches with numbness, weakness, or changes in vision or have migraine headaches with aura if you are over age 35
- breast cancer or any cancer that is sensitive to female hormones
- osteoporosis
- unexplained vaginal bleeding that has not been diagnosed. Your healthcare provider should check any unexplained vaginal bleeding to find out the cause.
- liver problems including liver disease
- smoke and are over 35 years old
- are taking medicines known as OATP1B1 inhibitors that are known or expected to significantly increase the blood levels of elagolix (an ingredient in Oriahnn). Ask your healthcare provider if you are not sure if you are taking this type of medicine.
- have had a serious allergic reaction to elagolix, estradiol, norethindrone acetate, or any of the ingredients in Oriahnn. Ask your healthcare provider if you are not sure.
- FD&C Yellow No.5 (tartrazine) is an ingredient in Oriahnn which may cause an allergic type reaction such as bronchial asthma in some patients who are also allergic to aspirin. See the end of this Medication Guide for a complete list of ingredients in Oriahnn.
What should I tell my healthcare provider before taking Oriahnn?
Before you take Oriahnn, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had:
- broken bones or other conditions that may cause bone problems.
- depression, mood swings, or suicidal thoughts or behavior.
- yellowing of the skin or eyes (jaundice) or jaundice caused by pregnancy (cholestasis of pregnancy).
- are scheduled for surgery. Oriahnn may increase your risk of blood clots after surgery. Your doctor may advise you to stop taking Oriahnn before you have surgery. If this happens, talk to your healthcare provider about when to restart Oriahnn after surgery.
- are pregnant or think you may be pregnant.
- are breastfeeding. It is not known if Oriahnn can pass into your breastmilk. Talk to your healthcare provider about the best way to feed your baby if you take Oriahnn.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Women on thyroid or cortisol replacement therapy may need increased doses of the hormone.
Know the medicines you take. Keep a list of your medicines with you to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Oriahnn?
- Take Oriahnn exactly as your healthcare provider tells you to take it.
- Your healthcare provider will give you a pregnancy test before you start taking Oriahnn or will have you start taking Oriahnn within 7 days after you start your period.
- Take 1 white and yellow Oriahnn capsule in the morning and 1 white and light blue Oriahnn capsule in the evening each day.
- Take Oriahnn at about the same time each morning and evening with or without food.
- If you take too much Oriahnn, call your healthcare provider or go to the nearest hospital emergency room right away.
If you miss a dose of Oriahnn (morning or evening capsules):
- Take the missed dose within 4 hours of the time that it was supposed to be taken. Then take the next dose at the usual time.
- If more than 4 hours have passed since you usually take the morning or evening dose, skip the missed dose. Take your next dose at the usual time.
- Do not take 2 doses to make up for the missed dose.
What should I avoid while taking Oriahnn?
- Avoid grapefruit and grapefruit juice during treatment with Oriahnn since they may affect the level of Oriahnn in your blood, which may increase side effects.
What are the possible side effects of Oriahnn?
Oriahnn may cause serious side effects including:
- See “What is the most important information I should know about Oriahnn?”
- suicidal thoughts, suicidal behavior, and worsening of mood. Oriahnn may cause suicidal thoughts or actions. Call your healthcare provider or get emergency medical help right away if you have any of these symptoms, especially if they are new, worse, or bother you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- other unusual changes in behavior or moodPay attention to any changes, especially sudden changes in your mood, behaviors, thoughts, or feelings.
- abnormal liver tests. Call your healthcare provider right away if you have any of these signs and symptoms of liver problems:
- jaundice
- dark amber-colored urine
- feeling tired (fatigue or exhaustion)
- nausea and vomiting
- generalized swelling
- right upper stomach area (abdomen) pain
- bruising easily
- high blood pressure. You should see your healthcare provider to check your blood pressure regularly.
- gallbladder problems (cholestasis), especially if you had cholestasis of pregnancy.
- increases in blood sugar, cholesterol and fat (triglyceride) levels.
- hair loss (alopecia). Hair loss and hair thinning can happen while taking Oriahnn and it can continue even after you stop taking Oriahnn. It is not known if this hair loss or hair thinning is reversible. Talk to your healthcare provider if this is a concern for you.
- changes in laboratory tests including thyroid and other hormone, cholesterol, and blood clotting tests.
The most common side effects of Oriahnn include: hot flushes, headache, fatigue, and irregular periods.
These are not all the possible side effects of Oriahnn. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Oriahnn
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Oriahnn for a condition for which it was not prescribed. Do not give Oriahnn to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Oriahnn that is written for health professionals.
How should I store Oriahnn?
- Store Oriahnn at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not keep medicine that is out of date or that you no longer need.
- Dispose of unused medicines through community take-back disposal programs when available. If no community take-back disposal program is available go to www.fda.gov/drugdisposal for information on how to dispose of Oriahnn the right way.
- Do not flush Oriahnn down the toilet.
Keep Oriahnn and all medicines out of the reach of children.
What are the ingredients in Oriahnn?
Yellow/White AM Capsule:
Active ingredient: elagolix, estradiol, norethindrone acetate.
Inactive ingredients: anhydrous sodium carbonate, polyethylene glycol 3350, crospovidone, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, purified water, lactose monohydrate, starch (corn), copovidone, talc, hypromellose, triacetin, and a gelatin capsule shell.
The capsule shell contains the following ingredients: FD&C Red #40, FD&C Yellow #5, FD&C Yellow #6, titanium dioxide, gelatin, and printing ink (shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water).
Light Blue/White PM Capsule
Active ingredient: elagolix.
Inactive ingredients: anhydrous sodium carbonate, polyethylene glycol 3350, crospovidone, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc, purified water, and a gelatin capsule shell.
The capsule shell contains the following ingredients: FD&C Blue #2, FDA/E172 yellow iron oxide, titanium dioxide, gelatin, and printing ink (shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water).
Label
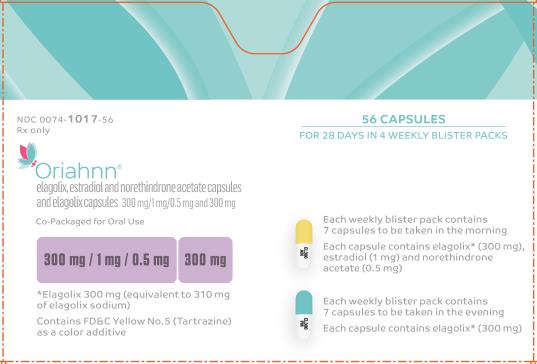
PRINCIPAL DISPLAY PANEL
- NDC 0074-1017-56
- Rx only
- 56 CAPSULES
- FOR 28 DAYS IN 4 WEEKLY BLISTER PACKS
- Oriahnn®
- elagolix, estadiol and norethindrone acetate capsules and elagolix capsules 300 mg/1 mg/0.5 mg and 300 mg
- Co-Packaged for Oral Use
- 300 mg / 1 mg / 0.5 mg 300mg
- *Elagolix 300 mg (equivalent to 310 mg of elagolix sodium)
- Contains FD&C Yellow No. 5 (Tartrazine) as a color additive
- Each weekly blister pack contains 7 capsules to be taken in the morning
- Each capsule contains elagolix* (300 mg), estradiol (1 mg) and norethindrone acetate (0.5 mg)
- Each weekly blister pack contains 7 capsules to be taken in the evening
- Each capsule contains elagolix* (300 mg)
PRINCIPAL DISPLAY PANEL
- NDC 0074-1017-14
- Rx only
- 14 CAPSULES
- FOR 7 DAYS
- Oriahnn™
- elagolix, estadiol and norethindrone acetate capsules and elagolix capsules 300 mg/1 mg/0.5 mg and 300 mg
- Co-Packaged for Oral Use

SRC: NLM .