Qvar Redihaler
Generic name: beclomethasone inhalation
Drug class: Inhaled corticosteroids
Medically reviewed by A Ras MD.
What is Qvar Redihaler?
Qvar Redihaler is a breath‑actuated inhaled prescription medicine used as a maintenance treatment for the prevention and control of asthma in people 4 years of age and older.
Qvar Redihaler is not used to relieve sudden breathing problems.
It is not known if Qvar Redihaler is safe and effective in children less than 4 years of age.
Description
The active component of QVAR REDIHALER 40 mcg Inhalation Aerosol and QVAR REDIHALER 80 mcg Inhalation Aerosol is beclomethasone dipropionate, USP, a corticosteroid having the chemical name 9-chloro-11ß,17,21-trihydroxy-16ß-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate. Beclomethasone dipropionate is a diester of beclomethasone, a synthetic corticosteroid chemically related to dexamethasone. Beclomethasone differs from dexamethasone in having a chlorine at the 9‑alpha carbon in place of a fluorine, and in having a 16‑beta-methyl group instead of a 16‑alpha-methyl group. Beclomethasone dipropionate is a white to creamy white, odorless powder with a molecular formula of C28H37ClO7 and a molecular weight of 521.1. Its chemical structure is:
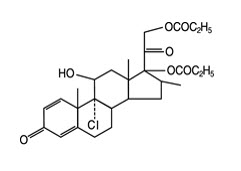
Chemical Structure
QVAR REDIHALER is a pressurized, breath‑actuated, metered‑dose aerosol with a dose counter intended for oral inhalation only. Each unit consists of a sealed breath‑actuated inhaler device enclosing a canister containing a solution of beclomethasone dipropionate in propellant HFA‑134a (1,1,1,2 tetrafluoroethane) and ethanol (0.85 g). QVAR REDIHALER 40 mcg delivers 40 mcg of beclomethasone dipropionate from the actuator mouthpiece and 50 mcg from the canister valve. QVAR REDIHALER 80 mcg delivers 80 mcg of beclomethasone dipropionate from the actuator mouthpiece and 100 mcg from the canister valve. Both products deliver 50 microliters (59 milligrams) of solution formulation as an aerosol from the canister valve with each actuation. The 40‑mcg canisters and the 80‑mcg canisters provide 120 inhalations each. Since the QVAR REDIHALER canister is fitted with a primeless valve, no priming actuations are required before use. For both products, an actuation was always triggered by a 20 L/min inspiratory flow rate.
Mechanism of Action
Beclomethasone dipropionate is a corticosteroid demonstrating potent anti‑inflammatory activity. The precise mechanism of corticosteroid action on asthma is not known. Corticosteroids have been shown to have multiple anti‑inflammatory effects, inhibiting both inflammatory cells (e.g., mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils) and release of inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines). These anti‑inflammatory actions of corticosteroids contribute to their efficacy in asthma.
Beclomethasone dipropionate is a prodrug that is rapidly activated by hydrolysis to the active monoester, 17‑monopropionate (17‑BMP). Beclomethasone‑17‑monopropionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 13 times that of dexamethasone, 6 times that of triamcinolone acetonide, 1.5 times that of budesonide and 25 times that of beclomethasone dipropionate. The clinical significance of these findings is unknown.
Studies in patients with asthma have shown a favorable ratio between topical anti‑inflammatory activity and systemic corticosteroid effects with recommended doses of QVAR REDIHALER.
Who should not use Qvar Redihaler?
Do not use Qvar Redihaler:
- to treat sudden severe symptoms of asthma.
- as a rescue inhaler.
- if you are allergic to beclomethasone dipropionate or any of the ingredients in Qvar Redihaler. See the below for a complete list of ingredients in Qvar Redihaler.
What should I tell my healthcare provider before using Qvar Redihaler?
Before using Qvar Redihaler, tell your healthcare provider about all of your medical conditions, including if you:
- are exposed to chickenpox or measles.
- have or have had tuberculosis (TB) or any untreated fungal, bacterial or viral infections, or eye infections caused by herpes.
- have weak bones (osteoporosis).
- have an immune system problem.
- have or have had eye problems, such as blurred vision, increased pressure in your eye (glaucoma) or cataracts.
- are pregnant or plan to become pregnant. It is not known if Qvar Redihaler will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Qvar Redihaler passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use Qvar Redihaler.
Tell your healthcare provider about all of the medications you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Qvar Redihaler?
Read the step-by-step instructions for using Qvar Redihaler at the end of this Patient Information guide.
- Use Qvar Redihaler exactly as your healthcare provider tells you to. Do not use Qvar Redihaler more often than it is prescribed.
- Do not shake the inhaler before using it. Especially, do not shake the inhaler with the cap open. This could cause the device to accidentally release medicine before you are ready to take it.
- You do not need to prime Qvar Redihaler.
- If your child needs to use Qvar Redihaler, watch your child closely to make sure your child uses the inhaler correctly.
- Do not change or stop using Qvar Redihaler or other asthma medicines used to treat your breathing problems unless your healthcare provider tells you to. Your healthcare provider will change your medicines as needed.
- You must use Qvar Redihaler regularly. It may take 2 to 4 weeks, or longer, after you start using Qvar Redihaler for your asthma symptoms to get better. Do not stop using Qvar Redihaler, even if you are feeling better, unless your healthcare provider tells you to.
- Qvar Redihaler comes in 2 strengths (40 and 80 mcg). Your healthcare provider has prescribed the strength that is best for you. Pay attention to the differences between Qvar Redihaler and your other inhaled medicines, including their prescribed use and the way they look.
- Qvar Redihaler does not relieve sudden asthma symptoms. Always have a rescue inhaler with you to treat sudden symptoms. Use your rescue inhaler if you have breathing problems between doses of Qvar Redihaler. If you do not have a rescue inhaler, call your healthcare provider to have a rescue inhaler prescribed for you.
- Rinse your mouth with water without swallowing after each dose of Qvar Redihaler. This will help lessen the chance of getting a yeast infection (thrush) in your mouth and throat.
- Do not spray Qvar Redihaler in your face or eyes. If you accidentally get Qvar Redihaler in your eyes, rinse your eyes with water and if redness or irritation continues, call your healthcare provider.
What should I avoid while taking Qvar Redihaler?
If you have not had, or have not been vaccinated against, chickenpox or measles, you should stay away from people who are infected.
What are the possible side effects of Qvar Redihaler?
Qvar Redihaler may cause serious side effects, including:
- fungal infections (thrush) in your mouth and throat. You may develop a yeast infection (Candida albicans) in your mouth and throat. Tell your healthcare provider if you have any redness or white colored patches in your mouth or throat. Rinse your mouth with water without swallowing after using Qvar Redihaler to help prevent an infection in your mouth or throat.
- worsening asthma or sudden asthma attacks. You should contact your healthcare provider right away if you do not get relief from your sudden asthma attacks, after using your rescue inhaler, during your treatment with Qvar Redihaler.
- reduced adrenal function (adrenal insufficiency). Adrenal insufficiency that can lead to death can happen when you stop taking oral corticosteroid medicines and start using inhaled corticosteroid medicines. Adrenal insufficiency can also happen in people who take higher doses of Qvar Redihaler than recommended over a long period of time. When your body is under stress such as from fever, trauma (such as a car accident), infection, or surgery, adrenal insufficiency can get worse. Signs and symptoms of adrenal insufficiency may include:
- feeling tired or exhausted (fatigue)
- lack of energy
- low blood pressure (hypotension)
- dizziness or feeling faint
- nausea and vomiting
- weakness
- immune system effects and a higher chance for infections. Tell your healthcare provider about any signs or symptoms of infection such as:
- fever
- chills
- pain
- feeling tired
- body aches
- nausea
- vomiting
- increased wheezing (bronchospasm) right after using Qvar Redihaler. Always have a rescue inhaler with you to treat sudden wheezing.
- serious allergic reactions. Stop using Qvar Redihaler and call your healthcare provider or get emergency medical help right away if you get any of the following signs or symptoms of a serious allergic reaction:
- hives
- swelling of your lips, tongue or face
- rash
- breathing problems
- slowed growth in children. Children should have their growth checked regularly while using Qvar Redihaler.
- lower bone density. This may be a problem for people who already have a higher chance for low bone density (osteoporosis).
- eye problems. If you have had glaucoma, cataracts or blurred vision in the past, you should have regular eye exams while using Qvar Redihaler.
The most common side effects of Qvar Redihaler include:
- yeast infection in the mouth (oral candidiasis)
- cold symptoms (upper respiratory tract infection)
- pain in the throat (oropharyngeal pain)
- pain or swelling in your nose and throat (nasopharyngitis)
- sinus irritation (sinusitis)
- hay fever (allergic rhinitis)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Qvar Redihaler. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Qvar Redihaler
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Qvar Redihaler for a condition for which it was not prescribed. Do not give Qvar Redihaler to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Qvar Redihaler that is written for health professionals.
How should I store Qvar Redihaler?
- Store Qvar Redihaler at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Your Qvar Redihaler canister should only be used with the Qvar Redihaler actuator. Do not use any other medicines in your Qvar Redihaler actuator.
- The contents of your Qvar Redihaler canister are under pressure. Do not puncture the Qvar Redihaler canister.
- Do not store your Qvar Redihaler canister near heat or a flame. Temperatures above 120ºF may cause the canister to burst.
- Do not throw your Qvar Redihaler canister into a fire or incinerator.
Keep Qvar Redihaler and all medicines out of the reach of children.
What are the ingredients in Qvar Redihaler?
Active ingredient: beclomethasone dipropionate
Inactive ingredients: propellant HFA-134a and ethanol
For more information, go to www.QVAR.com or call 1-888-483-8279.
Instructions for Use
When you are ready to use your Qvar Redihaler for the first time, remove the inhaler from the carton.
Important information:
- There is no button. You must close the white cap to prepare the inhaler with medicine before each inhalation.
- Do not shake. This breath-actuated device does not need to be shaken. This is not a press-and-breathe inhaler
- Qvar Redihaler does not need priming.
- Do not use a spacer or volume holding chamber with Qvar Redihaler.
- Always use the inhaler in the upright position (with the mouthpiece down).
- Once prepared, the inhaler will deliver 1 inhalation of medicine when you breathe in (inhale) through the mouthpiece. Your dose might require more than 1 inhalation.
- Do not open the white cap or leave it open unless you are ready for your next inhalation. If the cap has been opened for more than 2 minutes or left in the open position, you will need to close the white cap before use.
- Do not suddenly stop using your Qvar Redihaler. Contact your healthcare provider immediately if you stop using your Qvar Redihaler.
There are 2 main parts of your Qvar Redihaler including:
- the inhaler body with the mouthpiece. See Figure A.
- the white cap that covers the mouthpiece of the inhaler. See Figure A.

Figure A
About the Dose Counter
There is a dose counter in the back of the inhaler with a viewing window that shows you how many inhalations of medicine you have left. See Figure B.
- Your Qvar Redihaler contains 120 inhalations. See Figure B.
- The counter on the back of your inhaler shows how many inhalations you have left.
- When there are 20 inhalations left, the numbers in the dose counter will change to red and you should refill your prescription or ask your healthcare provider for another prescription.
- When the dose counter shows ‘0’, the background will turn solid red and your inhaler is empty. You should stop using the inhaler and throw it away. Do not put your inhaler into a fire or incinerator. See Figure B.

Figure B
Important:
- The white cap must be closed to prepare the inhaler before each inhalation or you will not receive your medicine. See Figure C.
- If the white cap is open, close the white cap to prepare your inhaler and look at the dose counter window to make sure that your inhaler is not empty. See Figure B.
- Do not open the cap until you are ready to take your inhalation.

Figure C
Using your Qvar Redihaler:
Step 1. Open the white cap
- Open the white cap. See Figure D.
- Breathe out fully.

Figure D
Remember:
- Do not open the cap until you are ready to take your inhalation.
- Never breathe out into the inhaler mouthpiece.
Step 2. Inhale 1 Time
- Place the mouthpiece in your mouth and close your lips around it so you form a good seal.
- Inhale deeply to release medication.
- Remove inhaler, hold breath for 5 to 10 seconds, then, breathe out slowly, away from the inhaler.

Figure E
Remember:
- Hold inhaler upright as you take your inhalation. See Figure E.
Step 3.Close the white cap
- Close the white cap after inhaling to prepare your next inhalation. See Figure F.

Figure F
If your healthcare provider has told you to take more than 1 inhalation per dose, make sure the white cap is closed and repeat steps 1-3.
After taking your prescribed number of inhalations, rinse your mouth with water without swallowing to help reduce the risk of a fungal infection (thrush) in your mouth.
How to store your Qvar Redihaler
- Store Qvar Redihaler at room temperature between 68 ºF to 77ºF (20ºC – 25ºC). Excursions between 59ºF and 86ºF (15ºC and 30ºC) are permitted. Do not use or store near heat or open flame. Exposure to temperatures above 120ºF (49ºC) may cause the canister to burst. Do not throw Qvar Redihaler into fire or an incinerator. Keep the white cap on the inhaler closed during storage.
- Keep your Qvar Redihaler inhaler dry and clean at all times.
- Keep your Qvar Redihaler and all medicines out of the reach of children.
- Throw away Qvar Redihaler when the dose counter displays ‘0,’ or after the expiration date on the package, whichever comes first.
Cleaning your Qvar Redihaler
- Do not wash or put any part of your Qvar Redihaler in water.
- Clean the mouthpiece of your Qvar Redihaler weekly with a clean, dry tissue or cloth.
Label
QVAR Redihaler® (beclomethasone dipropionate HFA) 40 mcg, 120 Metered Inhalations Carton Text
- NDC 59310-302-40
- QVAR
- RediHaler
- (beclomethasone dipropionate HFA)
- Breath-Actuated Inhalation Aerosol
- 40 mcg
- For oral inhalation only
- Lift side flap
- for instructions on
- how to use inhaler
- 120
- Metered
- Inhalations
- 10.6g NET CONTENT
PANEL PART 1 OF 2

40 mcg carton part 1
PANEL PART 2 OF 2

40 mcg part 2
QVAR RediHaler® (beclomethasone dipropionate HFA) 80 mcg, 120 Metered Inhalations Carton Text
- NDC 59310-304-80
- QVAR
- RediHaler
- (beclomethasone dipropionate HFA)
- Breath-Actuated Inhalation Aerosol
- 80 mcg
- For oral inhalation only
- Lift side flap
- for instructions on
- how to use inhaler
- 120
- Metered
- Inhalations
- 10.6g NET CONTENTS
PANEL PART 1 OF 2

80 mcg part 1
PANEL PART 2 OF 2
