Siklos
Generic name: hydroxyurea
Brand names: Droxia, Hydrea, Mylocel, Siklos
Drug class: Antimetabolites
Medically reviewed by A Ras MD.
What is Siklos?
Siklos is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises.
It is not known if Siklos is safe and effective in children less than 2 years of age.
Description
SIKLOS (hydroxyurea) is an antimetabolite that is available for oral use as functionally scored 100 mg film-coated tablet and functionally triple-scored 1,000 mg film-coated tablet containing 100 and 1,000 mg of hydroxyurea, respectively. Inactive ingredients include silicified microcrystalline cellulose, sodium stearyl fumarate, and film-coating agent amino methacrylate copolymer.
Hydroxyurea is a white crystalline powder. It has a molecular weight of 76.05. Its structural formula is:
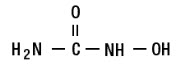
Mechanism of Action
The precise mechanism by which hydroxyurea produces its cytotoxic and cytoreductive effects is not known. However, various studies support the hypothesis that hydroxyurea causes an immediate inhibition of DNA synthesis by acting as a ribonucleotide reductase inhibitor, without interfering with the synthesis of ribonucleic acid or of protein.
The mechanisms by which SIKLOS produces its beneficial effects in patients with sickle cell Anemia (SCA) are uncertain. Known pharmacologic effects of SIKLOS that may contribute to its beneficial effects include increasing hemoglobin F levels in red blood cells (RBCs), decreasing neutrophils, increasing the water content of RBCs, increasing deformability of sickled cells, and altering the adhesion of RBCs to endothelium.
What is the most important information I should know about Siklos?
Siklos can cause serious side effects including:
- Low blood cell counts are common with Siklos, including low red blood cells, white blood cells, and platelets, and can be severe and life-threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and during treatment with Siklos. Your healthcare provider may change your dose or tell you to stop taking Siklos if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms:
- fever or chills
- body aches
- feeling very tired
- shortness of breath
- unusual headache
- bleeding or unexplained bruising
- Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking Siklos for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
- Siklos can harm your unborn baby.
For females taking Siklos who can become pregnant:- You should talk with your healthcare provider about the risks of Siklos to your unborn baby.
- You should use effective birth control during treatment with Siklos and for at least 6 months after treatment with Siklos.
- Your healthcare provider will perform a pregnancy test before you start treatment with Siklos. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- For males taking Siklos: Siklos can affect your sperm. If you have a female sexual partner who can become pregnant, you should use effective birth control during treatment with Siklos and for at least 6 months after treatment.
Siklos may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
See “What are the possible side effects of Siklos?” for more information about side effects.
Who should not take Siklos?
Do not take Siklos if you are allergic to hydroxyurea or any of the ingredients in Siklos. See the end of this Medication Guide for a list of the ingredients in Siklos.
What should I tell my healthcare provider before taking Siklos?
Before taking Siklos, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis
- have liver problems
- have human immunodeficiency virus (HIV) or take HIV medicines. Taking Siklos with certain HIV medicines can cause serious reactions and may lead to death.
- have increased levels of uric acid in your blood (hyperuricemia)
- have a history of receiving interferon therapy or are currently receiving interferon therapy
- have leg wounds or ulcers
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with SIKLOS.
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Siklos?”
- are breastfeeding or plan to breastfeed. It is not known if Siklos can pass into your breast milk. Do not breastfeed during treatment with Siklos.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Siklos?
Read the Instructions for Use that come with Siklos for step-by-step instructions on how to prepare a dose of Siklos. If you have any questions, talk to your healthcare provider or pharmacist.
- Take Siklos exactly as your healthcare provider tells you to take it.
- Take Siklos 1 time a day at the same time each day.
- Swallow the tablet with a glass of water.
- Siklos is supplied as 100 mg tablets and 1,000 mg tablets. The Siklos 1,000 mg tablet has three separation lines (score lines) and can be broken at these score lines to provide smaller doses. Each 1,000 mg tablet can be divided into 4 equal parts (each part is 250 mg).
- Do not break the Siklos 100 mg tablets into smaller parts.
- Your healthcare provider will tell you how many tablets or parts of a tablet you should take. If you are not able to swallow SIKLOS tablets, you can dissolve your prescribed dose in a small amount of water in a teaspoon and swallow right away.
- Siklos tablets must be handled with care. To decrease the risk of exposure, you or your caregivers should do the following when handling Siklos:
- Wear disposable gloves when handling Siklos or bottles containing Siklos. Wash your hands with soap and water before and after handling Siklos tablets or bottles containing Siklos.
- Avoid contact with crushed tablets. If contact with crushed tablets happens on the skin, wash the skin area right away and thoroughly with soap and water. If contact with crushed tablets happens in the eyes, flush the eyes thoroughly with water or isotonic eyewash used for that purpose for at least 15 minutes.
Powder spilled from the broken tablet should be wiped up with a damp disposable towel which must be thrown away in a closed container such as a plastic bag to avoid ingestion of powder by other people. The spill areas should then be cleaned using a detergent solution followed by clean water.
- If you take too much Siklos, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Siklos?
Siklos may cause serious side effects, including:
See “What is the most important information I should know about Siklos?”
- Skin ulcers, including leg ulcers have happened in people who take Siklos. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with Siklos if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take Siklos and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
The most common side effects of Siklos include:
- infections
- headache
- fever
- skin problems including:
- skin reactions
- dry skin
- changes in skin and nail color
- stomach and intestine (gastrointestinal) problems including:
- nausea
- constipation
- decrease in vitamin D
- weight gain
These are not all the possible side effects of Siklos.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Siklos
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Siklos for a condition for which it was not prescribed. Do not give Siklos to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Siklos that is written for health professionals.
How should I store Siklos?
- Store Siklos at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the Siklos bottle tightly closed.
- Broken 1,000 mg Siklos tablets must be stored in the bottle and must be used within three months.
Keep Siklos and all medicines out of the reach of children.
What are the ingredients in Siklos?
Active ingredient: hydroxyurea
Inactive ingredients: silicified microcrystalline cellulose, sodium stearyl fumarate, and film-coating agent amino methacrylate copolymer.
Label
PRINCIPAL DISPLAY PANEL – 1,000 MG TABLET BOTTLE CARTON
- NDC 71770-120-30
- Siklos® 1,000 mg
(hydroxyurea)
tablets - 1,000 mg per tablet
- ATTENTION
PHARMACIST:
Each patient is
required to receive
the enclosed
Medication Guide - 30 tablets
- addmedica

PRINCIPAL DISPLAY PANEL – 100 MG TABLET BOTTLE LABEL
- Siklos®
100 mg
(hydroxyurea)
100 mg per tablet
60 tablets - NDC 71770-105-60
Store at 20°C to 25°C (68°F to 77°F)
Wear disposable gloves when handling Siklos®or bottles containing Siklos®
ATTENTION PHARMACIST:
Each patient is required to
receive the enclosed
Medication Guide - RX Only.
addmedica


SRC: NLM .