Soltamox
Generic name: tamoxifen
Drug classes: Hormones / antineoplastics, Selective estrogen receptor modulators
Medically reviewed by A Ras MD.
What is Soltamox?
Soltamox is a prescription medicine used to treat adults with estrogen receptor-positive breast cancer that has spread to other parts of the body (metastatic), adults with early-stage estrogen receptor-positive breast cancer after surgery and radiation for breast cancer,
It is also used to reduce the chance of developing breast cancer in the other breast in adults after surgery and radiation for breast cancer to reduce the risk of invasive breast cancer in adult women with DCIS, after breast surgery and radiation treatment to reduce the risk of getting breast cancer in women with a high risk of getting breast cancer
It is not known if Soltamox is safe and effective in children.
Description
SOLTAMOX (tamoxifen citrate) oral solution is for oral administration. Tamoxifen citrate is an estrogen agonist/antagonist.
Chemically, tamoxifen is the trans-isomer of a triphenylethylene derivative. The chemical name is (Z)2-[4-(1,2- diphenyl-1-butenyl)phenoxy]-N,N-dimethylethanamine 2-hydroxy-1,2,3- propanetricarboxylate (1:1). The structural formula, empirical formula, and molecular weight are as follows:
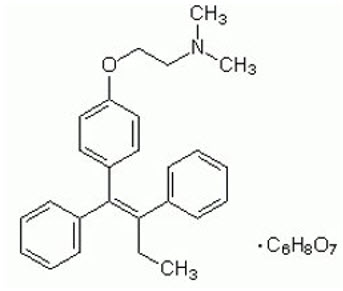
|
Tamoxifen citrate has a pKa′ of 8.85. The equilibrium solubility in water at 37°C is 0.5 mg/mL, and is 0.2 mg/mL in 0.02 N HCl at 37°C.
Each 10 mL of SOLTAMOX oral solution contains 20 mg tamoxifen, equivalent to 30.4 mg tamoxifen citrate. The oral solution is a sugar-free, clear colorless liquid, with licorice and aniseed odor and taste supplied in a 150 mL bottle with a dosing cup. SOLTAMOX oral solution contains the following inactive ingredients: ethanol, glycerol, propylene glycol, sorbitol solution, licorice flavor, aniseed flavor, purified water.
Mechanism of Action
Tamoxifen is an estrogen agonist/antagonist. Tamoxifen competes with estrogen for binding to the estrogen receptor, which can result in a decrease in estrogen receptor signaling-dependent growth in breast tissue. Tamoxifen has demonstrated antitumor activity against human breast cancer cell lines xenografted in mice. The drug has been shown to inhibit the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and to cause the regression of already established DMBA-induced tumors.
What is the most important information I should know about Soltamox?
Soltamox can cause serious side effects, including:
- Uterine cancer. Cancer of the lining of the uterus (endometrial adenocarcinoma) or cancer of the body of the uterus (uterine sarcoma) may happen more often in women who take Soltamox and can lead to death. People who take or have taken Soltamox should have a gynecologic exam every year.
Soltamox can also cause other non-cancer effects on the uterus, including:- overgrowth of the lining of the uterus (hyperplasia) and uterine polyps
- endometriosis
- fibroids
- irregular menstrual periods or no menstrual periods (amenorrhea)Tell your healthcare provider right away if you get any of the following symptoms during or after treatment with Soltamox:
- irregular menstrual periods
- abnormal vaginal bleeding
- a change in your vaginal discharge
- pelvic pain or pressure
- Blood clots in your veins or lungs. Blood clots in your veins or lungs may happen more often in people who take Soltamox and can lead to death, especially in people who take Soltamox during their treatment with chemotherapy.
Tell your healthcare provider right away if you get any of the following symptoms of a blood clot during treatment with Soltamox:- sudden chest pain, shortness of breath, or coughing up blood
- pain, tenderness, or swelling in one or both of your legs
- Stroke.Stroke can cause serious problems and can lead to death. Get medical help right away if you get any of these symptoms of a stroke:
- sudden weakness, tingling, or numbness of your face, arm or leg, especially on one side of your body
- sudden confusion, trouble speaking or understanding
- sudden trouble seeing in one or both eyes
- sudden trouble walking, dizziness, loss of balance or coordination
- sudden severe headache with no known cause
Who should not take Soltamox?
Do not take Soltamox if you:
- have had a serious allergic reaction to tamoxifen or any other ingredient in Soltamox. Ask your healthcare provider if you are not sure.
- have DCIS or a high-risk of breast cancer, and you
- need to take the blood thinner medicine warfarin, or
- have a history of a blood clot in your veins or lungs
What should I tell my healthcare provider before taking Soltamox?
Before taking Soltamox, tell your healthcare provider about all of your medical conditions, including if you:
- have uterine cancer or other problems with your uterus. See “What is the most important information I should know about Soltamox?”
- have irregular menstrual periods, no menstrual periods, or abnormal vaginal bleeding
- have or had a blood clot in your veins or lungs
- have had a stroke
- are pregnant or plan to become pregnant. Soltamox can harm your unborn baby. You should not become pregnant during treatment with Soltamox or within 2 months after you stop taking it.
Females who are able to become pregnant:- Before you start treatment with Soltamox, your healthcare provider should do a pregnancy test to make sure that you are not pregnant.
- You should use effective birth control (contraception) during treatment with Soltamox and for 2 months after the last dose. Talk to your healthcare provider about birth control options that you may use during this time. You should not use hormonal contraceptives such as birth control pills, patches, implants, or IUDs that contain hormones.
- Tell your healthcare provider right away if you become pregnant during treatment with Soltamox.
- are breastfeeding or plan to breastfeed. It is not known if Soltamox passes into your breast milk. You should not breastfeed during treatment with Soltamox and for 3 months after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
Before taking Soltamox, you and your healthcare provider should talk about the possible benefits and risks.
- The benefits and risks are different for women at high risk for breast cancer and women with breast cancer that is only inside the milk ducts (ductal carcinoma in-situ or DCIS) than for women who have been diagnosed with invasive breast cancer.
- For most people who already have invasive breast cancer, the benefits of Soltamox are greater than the risks.
- Talk to your healthcare provider about which risks may affect you.
How should I take Soltamox?
- Take Soltamox exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much Soltamox to take and when to take it.
- Your healthcare provider will decide how long you should continue to take Soltamox, depending on your medical condition.
- Do not stop taking Soltamox without first talking with your healthcare provider.
- Use the dosing cup that comes with Soltamox to measure the correct amount for each dose. Ask your healthcare provider or pharmacist if you are not sure how to measure your dose.
- If you forget a dose of Soltamox, take it when you remember, then take the next dose at your usual time. If it is almost time for your next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time. If you are not sure about your dosing, call your healthcare provider.
- If you take too much Soltamox, call your healthcare provider right away.
What are the possible side effects of Soltamox?
Soltamox can cause serious side effects, including:
- See “What is the most important information I should know about Soltamox?”
- Liver problems, including liver cancer. Soltamox can cause changes in liver function blood tests, and sometimes can cause liver cancer and other serious liver problems that can lead to death. Your healthcare provider should do blood tests to check you for these problems during treatment with Soltamox.
- Other cancers.
- High levels of calcium in the blood (hypercalcemia). People with breast cancer that has spread to their bones may develop hypercalcemia. Hypercalcemia can happen within a few weeks after you start taking Soltamox. Your healthcare provider should check you for this problem. If it is severe, your healthcare provider may tell you to stop taking Soltamox.
- Decreased blood cell counts. Soltamox can cause a decrease in platelet counts, red blood cell counts, and white blood cell counts that can be severe. Your healthcare provider should check your blood counts during treatment with Soltamox.
- Eye problems. Soltamox can increase your chance of developing cataracts and other eye problems. Tell your healthcare provider about any changes in your vision or other eye symptoms during treatment with Soltamox.
The most common side effects of Soltamox include:
- hot flashes
- mood changes
- vaginal discharge
- vaginal bleeding.”What is the most important information I should know about Soltamox?”
- nausea
- swelling (fluid retention)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Soltamox.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA- 1088.
General information about the safe and effective use of Soltamox
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Soltamox for a condition for which it was not prescribed. Do not give Soltamox to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Soltamox that is written for health professionals.
How should I store Soltamox?
- Store Soltamox at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze or refrigerate.
- Keep Soltamox in the original bottle to protect it from light.
- Keep the bottle tightly closed.
- Use within 3 months of opening. Throw away any Soltamox remaining in the bottle after 3 months.
Keep Soltamox and all medicines out of the reach of children.
What are the ingredients in Soltamox?
Active ingredient: tamoxifen citrate
Inactive ingredients: ethanol, glycerol, propylene glycol, sorbitol solution, licorice flavor, aniseed flavor, purified water.
Label
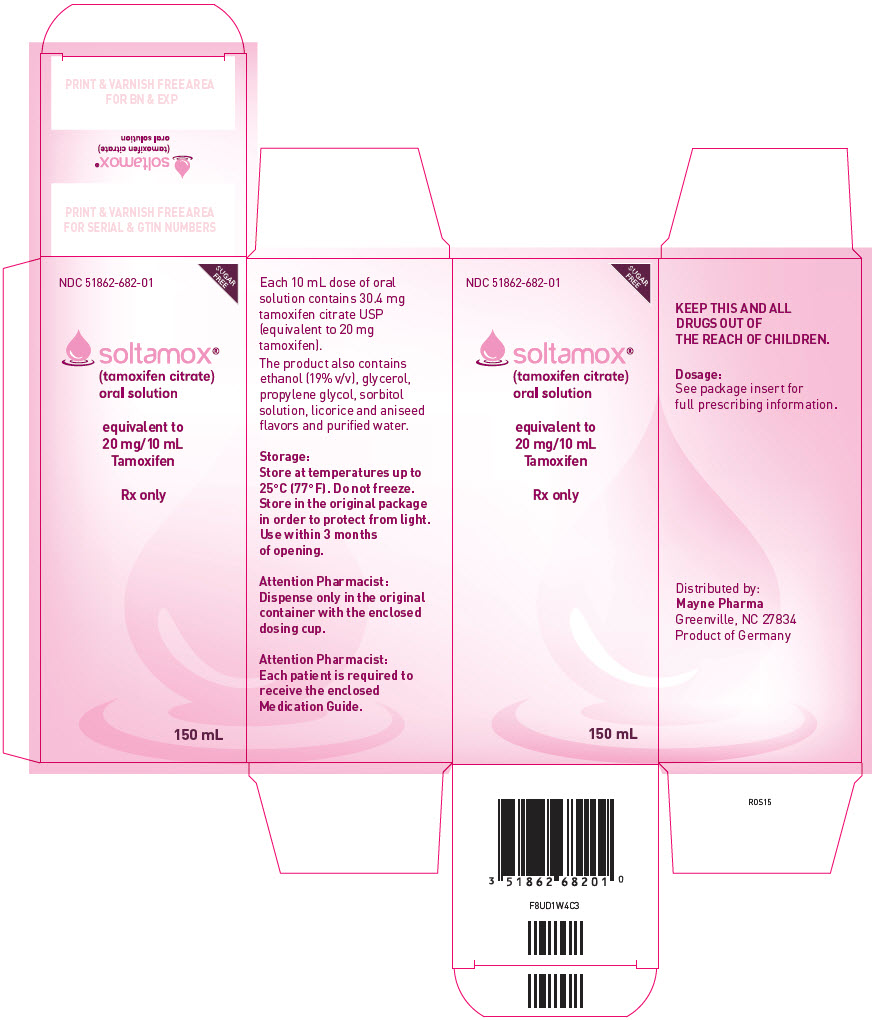
PRINCIPAL DISPLAY PANEL – 150 ML CONTAINER CARTON
- NDC 51862-682-01
- SUGAR
FREE - soltamox®
(tamoxifen citrate)
oral solution - equivalent to
20 mg/10 mL
Tamoxifen - Rx only
- 150 mL
SRC: NLM .