Selzentry
Generic name: maraviroc
Drug class: Chemokine receptor antagonist
Medically reviewed by A Ras MD.
What is Selzentry?
Selzentry is a prescription HIV-1 (Human Immunodeficiency Virus type 1) medicine used with other antiretroviral medicines to treat CCR5-tropic HIV-1 infection in people 2 years of age and older weighing at least 22 lb (10 kg). HIV-1 is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
Use of Selzentry is not recommended in people with dual/mixed or CXCR4‑tropic HIV‑1.
The safety and effectiveness of Selzentry has not been established in children younger than 2 years of age.
Description
SELZENTRY (maraviroc) is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Blocking this interaction prevents CCR5-tropic HIV-1 entry into cells.
SELZENTRY film-coated tablets for oral administration contain 25, 75, 150, or 300 mg of maraviroc and the following inactive ingredients: dibasic calcium phosphate (anhydrous), magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film coat (Opadry II Blue [85G20583]) contains FD&C blue #2 aluminum lake, soya lecithin, polyethylene glycol (macrogol 3350), polyvinyl alcohol, talc, and titanium dioxide.
SELZENTRY oral solution contains 20 mg per mL of maraviroc and the following inactive ingredients: citric acid (anhydrous), purified water, sodium benzoate, sodium citrate dihydrate, strawberry flavoring (501440T), and sucralose.
Maraviroc is chemically described as 4,4-difluoro-N-{(1S)-3–[exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl}cyclohexanecarboxamide.
The molecular formula is C29H41F2N5O and the structural formula is:
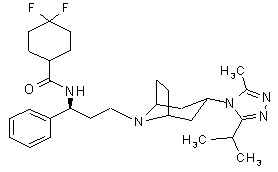
Maraviroc is a white to pale-colored powder with a molecular weight of 513.67. It is highly soluble across the physiological pH range (pH 1.0 to 7.5).
What is the most important information I should know about Selzentry?
Selzentry can cause serious side effects including serious liver problems (liver toxicity). An allergic reaction may happen before liver problems occur. Stop taking Selzentry and call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:
- an itchy rash on your body (allergic reaction)
- your skin or the white part of your eyes turns yellow (jaundice)
- dark or “tea-colored” urine
- vomiting
- pain, aching, or tenderness on the right side of your stomach area
Your healthcare provider will do blood tests to check your liver before you begin treatment with Selzentry and as needed during treatment, and if you get a severe rash, signs and symptoms of liver problems, or an allergic reaction during treatment with Selzentry.
Who should not take Selzentry?
Do not take Selzentry if you:
- have severe kidney problems or are on hemodialysis and are also taking certain other medications.
What should I tell my healthcare provider before taking Selzentry?
Before you take Selzentry, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had liver problems including hepatitis B or C virus infection.
- have heart problems.
- have kidney problems.
- have low blood pressure or take medicines to lower blood pressure.
- are pregnant or plan to become pregnant. It is not known if Selzentry may harm your unborn baby.
Pregnancy Registry. There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Selzentry. You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby. Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may interact with Selzentry. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Selzentry.
Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Selzentry with other medicines. Your healthcare provider may need to change your dose of Selzentry when you take it with certain medicines.
- You should not take Selzentry if you also take St. John’s wort (Hypericum perforatum).
How should I take Selzentry?
- Take Selzentry exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Selzentry without first talking with your healthcare provider.
- If you miss a dose of Selzentry, take it as soon as you remember. Do not take 2 doses at the same time. If you are not sure about your dosing, call your healthcare provider.
- Stay under the care of a healthcare provider while taking Selzentry.
- Swallow Selzentry tablets whole. Do not chew the tablets.
- Selzentry may be taken with or without food.
- For children aged 2 years and older and weighing at least 22 lb (10 kg), your healthcare provider will prescribe a dose of Selzentry based on your child’s body weight and other medicines they are taking.
- Tell your healthcare provider if your child has trouble swallowing tablets. Selzentry comes as tablets or as a liquid (oral solution).
- Selzentry oral solution should be given with the supplied press-in bottle adapter and oral dosing syringe. See the Instructions for Use that comes with Selzentry oral solution for information about the right way to take a dose.
- Do not run out of Selzentry. The virus in your blood may increase and the virus in your blood may become harder to treat. When your supply starts to run low, get more from your healthcare provider or pharmacy.
- If you take too much Selzentry, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Selzentry?
- Selzentry can cause serious side effects including:
- See “What is the most important information I should know about Selzentry?”
- Serious skin rash and allergic reactions. Severe and potentially life-threatening skin reactions and allergic reactions have been reported in some patients taking Selzentry. If you develop a rash with any of the following symptoms, stop using Selzentry and contact your doctor right away:
- fever
- generally ill feeling
- muscle aches
- blisters or sores in your mouth
- blisters or peeling of the skin
- redness or swelling of the eyes
- swelling of the mouth or face or lips
- problems breathing
- yellowing of the skin or whites of your eyes
- dark or tea-colored urine
- pain, aching, or tenderness on the right side below the ribs
- loss of appetite
- nausea / vomiting
- Heart problems including heart attack.
- Low blood pressure when standing up (postural hypotension) can cause dizziness or fainting. You should avoid driving or operating heavy machinery if you have dizziness while taking Selzentry.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you develop new symptoms after you start taking Selzentry.
- Possible chance of infection or cancer. Selzentry affects other immune system cells and therefore may possibly increase your chance for getting other infections or cancer.
The most common side effects of Selzentry in adults include colds and cold-like symptoms, cough, fever, rash, bloating and gas, indigestion, constipation, and dizziness.
The most common side effects of Selzentry in children include vomiting, abdominal pain, diarrhea, nausea, and dizziness.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Selzentry. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of Selzentry
Medicines are sometimes prescribed for purposes other than those mentioned in a Medication Guide. Do not use Selzentry for a condition for which it was not prescribed. Do not give Selzentry to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for the information about Selzentry that is written for health professionals.
For more information go to WWW.SELZENTRY.COM.
How should I store Selzentry?
- Store Selzentry tablets and oral solution at room temperature between 68°F to 77°F (20°C to 25°C).
- Throw away any unused oral solution 60 days after first opening the bottle.
Keep Selzentry and all medicines out of the reach of children.
What are the ingredients in Selzentry?
Active ingredient: maraviroc
Inactive ingredients:
Tablets: Dibasic calcium phosphate (anhydrous), magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. Tablet film-coating contains: FD&C blue #2 aluminum lake, soya lecithin, polyethylene glycol (macrogol 3350), polyvinyl alcohol, talc, and titanium dioxide.
Oral Solution: Citric acid, purified water, sodium benzoate, sodium citrate dihydrate, strawberry flavoring (501440T), and sucralose.
Label
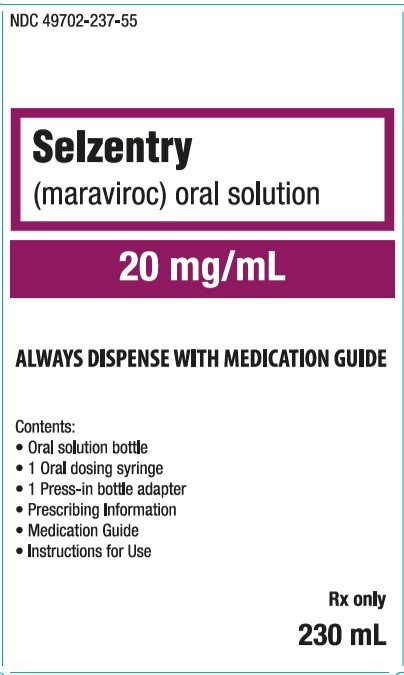
PRINICIPAL DISPLAY PANEL
- NDC 49702-237-55
- Selzentry
- (maraviroc) oral solution
- 20 mg/mL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
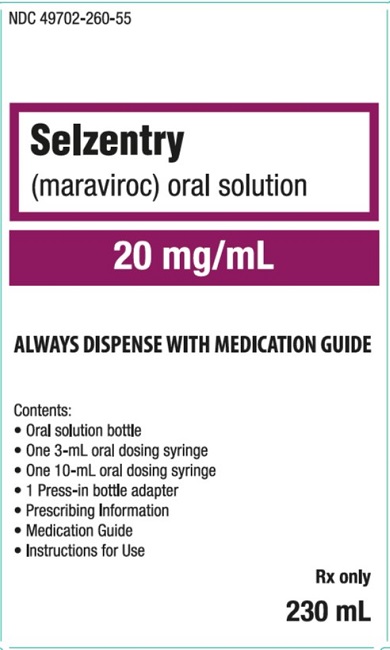
PRINCIPAL DISPLAY PANEL
- NDC 49702-260-55
- Selzentry
- (maraviroc) oral solution
- 20 mg/mL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
SRC: NLM .