Skyla
Generic name: levonorgestrel intrauterine system
Drug classes: Contraceptives, Progestins
Medically reviewed by A Ras MD.
What is Skyla?
Skyla is a hormone-releasing system placed in your uterus by your healthcare provider to prevent pregnancy for up to 3 years. Skyla can be removed by your healthcare provider at any time. Skyla can be used whether or not you have given birth to a child.
Skyla is a small, flexible plastic T-shaped system that slowly releases a progestin hormone called levonorgestrel (LNG) that is often used in birth control pills. Because Skyla releases LNG into your uterus, only small amounts of the hormone enter your blood. Skyla does not contain estrogen.
Two thin threads are attached to the stem (lower end) of Skyla. The threads are the only part of Skyla you can feel when Skyla is in your uterus; however, unlike a tampon string, the threads do not extend outside your body.
What if I need birth control for more than 3 years?
Skyla must be removed after 3 years. Your healthcare provider can place a new Skyla during the same office visit if you choose to continue using Skyla.
What if I want to stop using Skyla?
Skyla is intended for use up to 3 years but you can stop using Skyla at any time by asking your healthcare provider to remove it. You could become pregnant as soon as Skyla is removed, so you should use another method of birth control if you do not want to become pregnant. Talk to your healthcare provider about the best birth control methods for you, because your new method may need to be started 7 days before Skyla is removed to prevent pregnancy.
What if I change my mind about birth control and want to become pregnant in less than 3 years?
Your healthcare provider can remove Skyla at any time. You may become pregnant as soon as Skyla is removed. About 3 out of 4 women who want to become pregnant will become pregnant sometime in the first year after Skyla is removed.
How does Skyla work?
Skyla may work in several ways including thickening cervical mucus, inhibiting sperm movement, reducing sperm survival, and thinning the lining of your uterus. It is not known exactly how these actions work together to prevent pregnancy.
How well does Skyla work for contraception?
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.

Who might use Skyla?
You might choose Skyla if you:
- Want long-term birth control that provides a low chance of getting pregnant (less than 1 in 100)
- Want birth control that works continuously for up to 3 years
- Want birth control that is reversible
- Want a birth control method that you do not need to take daily
- Are willing to use a birth control method that is placed in the uterus
- Want birth control that does not contain estrogen
Description
Skyla (levonorgestrel-releasing intrauterine system) contains 13.5 mg of LNG, a progestin, and is intended to provide an initial release rate of approximately14 mcg/day of LNG after 24 days.
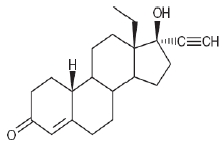
Levonorgestrel USP, (-)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one, the active ingredient in Skyla, has a molecular weight of 312.4, a molecular formula of C21H28O2, and the following structural formula:
11.1 Skyla
Skyla consists of a T-shaped polyethylene frame (T-body) with a steroid reservoir (hormone elastomer core) around the vertical stem. The white T-body has a loop at one end of the vertical stem and two horizontal arms at the other end. The reservoir consists of a whitish or pale yellow cylinder, made of a mixture of LNG and silicone (polydimethylsiloxane), containing a total of 13.5 mg LNG. The reservoir is covered by a semi-opaque silicone membrane, composed of polydimethylsiloxane and colloidal silica. A ring composed of 99.95% pure silver is located at the top of the vertical stem close to the horizontal arms and is visible by ultrasound. The polyethylene of the T-body is compounded with barium sulfate, which makes it radiopaque. A monofilament brown polyethylene removal thread is attached to a loop at the end of the vertical stem of the T-body. The polyethylene of the removal thread contains iron oxide as a colorant (see Figure 10).
The components of Skyla, including its packaging, are not manufactured using natural rubber latex.
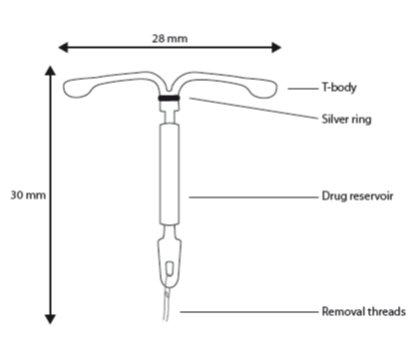
Figure 10. Skyla
11.2 Inserter
Skyla is packaged sterile within an inserter. The inserter (Figure 11), which is used for insertion of Skyla into the uterine cavity, consists of a symmetric two-sided body and slider that are integrated with flange, lock, pre-bent insertion tube and plunger. The outer diameter of the insertion tube is 3.8 mm. The vertical stem of Skyla is loaded in the insertion tube at the tip of the inserter. The arms are pre-aligned in the horizontal position. The removal threads are contained within the insertion tube and handle. Once Skyla has been placed, the inserter is discarded.
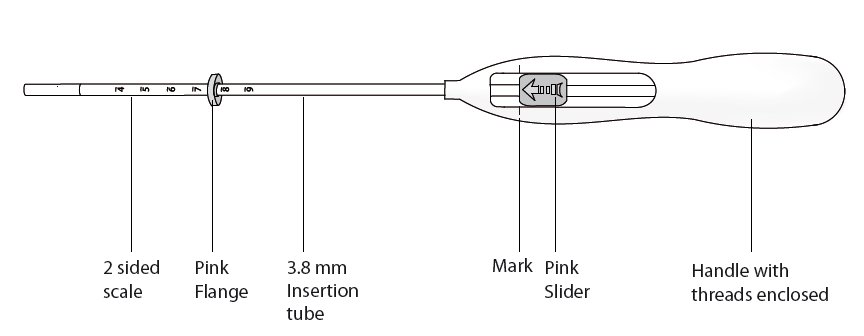
Figure 11. Diagram of Inserter
Mechanism of Action
The local mechanism by which continuously released LNG contributes to the contraceptive effectiveness of Skyla has not been conclusively demonstrated. Studies of Skyla and similar LNG IUS prototypes have suggested several mechanisms that prevent pregnancy: thickening of cervical mucus preventing passage of sperm into the uterus, inhibition of sperm capacitation or survival, and alteration of the endometrium.
What is the most important information I should know about Skyla?
Skyla does not protect against HIV infection (AIDS) and other sexually transmitted infections (STIs).
Who should not take Skyla?
Do not use Skyla if you:
- Are or might be pregnant; Skyla cannot be used as an emergency contraceptive
- Have had a serious pelvic infection called pelvic inflammatory disease (PID) unless you have had a normal pregnancy after the infection went away
- Have an untreated pelvic infection now
- Have had a serious pelvic infection in the past 3 months after a pregnancy
- Can get infections easily. For example, if you:
- Have multiple sexual partners or your partner has multiple sexual partners
- Have problems with your immune system
- Abuse intravenous drugs
- Have or suspect you might have cancer of the uterus or cervix
- Have bleeding from the vagina that has not been explained
- Have liver disease or a liver tumor
- Have breast cancer or any other cancer that is sensitive to progestin (a female hormone), now or in the past
- Have an intrauterine device in your uterus already
- Have a condition of the uterus that changes the shape of the uterine cavity, such as large fibroid tumors
- Are allergic to levonorgestrel, silicone, polyethylene, silver, silica, barium sulfate or iron oxide
What should I tell my healthcare provider before taking Skyla?
Before having Skyla placed, tell your healthcare provider if you:
- Have any of the conditions listed above
- Have had a heart attack
- Have had a stroke
- Were born with heart disease or have problems with your heart valves
- Have problems with blood clotting or take medicine to reduce clotting
- Have high blood pressure
- Recently had a baby or are breastfeeding
- Have severe migraine headaches
How should I use Skyla?
Skyla is placed by your healthcare provider during an in-office visit.
First, your healthcare provider will examine your pelvis to find the exact position of your uterus. Your healthcare provider will then clean your vagina and cervix with an antiseptic solution and slide a slim plastic tube containing Skyla into your uterus. Your healthcare provider will then remove the plastic tube, and leave Skyla in your uterus. Your healthcare provider will cut the threads to the right length. Placement takes only a few minutes.
You may experience pain, bleeding or dizziness during and after placement. If your symptoms do not pass within 30 minutes after placement, Skyla may not have been placed correctly. Your healthcare provider will examine you to see if Skyla needs to be removed or replaced.
What are the possible side effects of Skyla?
Skyla can cause serious side effects, including:
- Ectopic pregnancy and intrauterine pregnancy risks. There are risks if you become pregnant while using Skyla (see “What if I become pregnant while using Skyla?”).
- Life-threatening infection. Life-threatening infection can occur within the first few days after Skyla is placed. Call your healthcare provider immediately if you develop severe pain or fever shortly after Skyla is placed.
- Pelvic inflammatory disease (PID). Some IUD users get a serious pelvic infection called pelvic inflammatory disease. PID is usually sexually transmitted. You have a higher chance of getting PID if you or your partner has sex with other partners. PID can cause serious problems such as infertility, ectopic pregnancy or pelvic pain that does not go away. PID is usually treated with antibiotics. More serious cases of PID may require surgery. A hysterectomy (removal of the uterus) is sometimes needed. In rare cases, infections that start as PID can even cause death.
Tell your healthcare provider right away if you have any of these signs of PID: long-lasting or heavy bleeding, unusual vaginal discharge, low abdominal (stomach area) pain, painful sex, chills, or fever.
- Perforation. Skyla may become attached to (embedded) or go through the wall of the uterus. This is called perforation. If this occurs, Skyla may no longer prevent pregnancy. If perforation occurs, Skyla may move outside the uterus and can cause internal scarring, infection, or damage to other organs, and you may need surgery to have Skyla removed. The risk of perforation is increased if Skyla is inserted while you are breastfeeding.
Common side effects of Skyla include:
- Pain, bleeding or dizziness during and after placement. If these symptoms do not stop 30 minutes after placement, Skyla may not have been placed correctly. Your healthcare provider will examine you to see if Skyla needs to be removed or replaced.
- Expulsion. Skyla may come out by itself. This is called expulsion. Expulsion occurs in about 3 out of 100 women. You may become pregnant if Skyla comes out. If you think that Skyla has come out, use a backup birth control method like condoms and spermicide and call your healthcare provider.
- Missed menstrual periods. About 1 out of 16 women stop having periods after 1 year of Skyla use. If you do not have a period for 6 weeks during Skyla use, call your healthcare provider. When Skyla is removed, your menstrual periods should return.
- Changes in bleeding. You may have bleeding and spotting between menstrual periods, especially during the first 3–6 months. Sometimes the bleeding is heavier than usual at first. However, the bleeding usually becomes lighter than usual and may be irregular. Call your healthcare provider if the bleeding remains heavier than usual or increases after it has been light for a while.
- Cysts on the ovary. About 14 out of 100 women using Skyla develop a cyst on the ovary. These cysts usually disappear on their own in two to three months. However, cysts can cause pain and sometimes cysts will need surgery.
This is not a complete list of possible side effects with Skyla. For more information, ask your healthcare provider. Tell your healthcare provider if you have any side effect that bothers you or does not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to the manufacturer at 1-888-842-2937 or www.fda.gov/medwatch.
After Skyla has been placed, when should I call my healthcare provider?
If Skyla is accidentally removed and you had vaginal intercourse within the preceding week, you may be at risk of pregnancy, and you should talk to a healthcare provider.
Call your healthcare provider if you have any concerns about Skyla. Be sure to call if you:
- Think you are pregnant
- Have pelvic pain, abdominal pain, or pain during sex
- Have unusual vaginal discharge or genital sores
- Have unexplained fever, flu-like symptoms or chills
- Might be exposed to sexually transmitted infections (STIs)
- Are concerned that Skyla may have been expelled (came out)
- Cannot feel Skyla’s threads
- Develop very severe or migraine headaches
- Have yellowing of the skin or whites of the eyes. These may be signs of liver problems.
- Have had a stroke or heart attack
- Become HIV positive or your partner becomes HIV positive
- Have severe vaginal bleeding or bleeding that concerns you
General information about the safe and effective use of Skyla
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. You can ask your healthcare provider for information about Skyla that is written for healthcare providers.
For more information call 1-888-842-2937.
How should I store Skyla?
Store at 25°C (77°F); with excursions permitted between 15–30°C (59–86°F)
What are the ingredients in Skyla?
Active ingredients: Levonorgestrel
Inactive ingredients: Dimethicone, Barium Sulfate
Label
PRINCIPAL DISPLAY PANEL
- Skyla (levonorgestrel-releasing intrauterine system) Carton
- NDC 50419-422-01
- 1 Sterile Unit
- IMPORTANT: To be inserted in the uterus by or under the supervision of a licensed clinician. See physician insert for detailed instructions for use.
- Skyla
- (levonorgestrel-releasing intrauterine system)
- Rx only
- — 13.5 mg levonorgestrel
- — 1 sterile unit
- — intrauterine use

SRC: NLM .