Sutent
Generic name: sunitinib
Drug classes: Multikinase inhibitors, VEGF/VEGFR inhibitors
Medically reviewed by A Ras MD.
What is Sutent?
Sutent is a prescription medicine used to treat a rare cancer of the stomach, bowel, or esophagus called gastrointestinal stromal tumor (GIST) and when you have taken the medicine imatinib mesylate (Gleevec) and it did not stop the cancer from growing, or you cannot take imatinib mesylate (Gleevec).
It is also used in advanced kidney cancer (advanced renal cell carcinoma or RCC), adults with kidney cancer that has not spread (localized), and who are at high risk of RCC coming back again after having kidney surgery, a type of pancreatic cancer called pancreatic neuroendocrine tumors (pNET), that has progressed and cannot be treated with surgery.
It is not known if Sutent is safe and effective in children.
Description
Sunitinib is a kinase inhibitor present in SUTENT capsules as the malate salt. Sunitinib malate is described chemically as (2S)-2-hydroxybutanedoic acid with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (1:1). The molecular formula is C22H27FN4O2 ∙ C4H6O5 and the molecular weight is 532.6 Daltons. The chemical structure of sunitinib malate is:
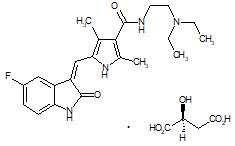
Sunitinib malate is a yellow to orange powder with a pKa of 8.95. The solubility of sunitinib malate in aqueous media over the range pH 1.2 to pH 6.8 is in excess of 25 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7 is 5.2.
SUTENT (sunitinib malate) capsules are supplied as printed hard shell capsules containing 12.5 mg, 25 mg, 37.5 mg or 50 mg of sunitinib (equivalent to 16.7 mg, 33.4 mg, 50.1 mg, or 66.8 mg of sunitinib malate, respectively). The capsules contain the following inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, and povidone (K-25). The orange gelatin capsule shells contain titanium dioxide and red iron oxide; the caramel gelatin capsule shells contain titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide; and the yellow gelatin capsule shells contain titanium dioxide and yellow iron oxide. The white printing ink contains shellac, propylene glycol, sodium hydroxide, povidone, and titanium dioxide and the black printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide.
Mechanism of Action
Sunitinib is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs), some of which are implicated in tumor growth, pathologic angiogenesis, and metastatic progression of cancer. Sunitinib was evaluated for its inhibitory activity against a variety of kinases (>80 kinases) and was identified as an inhibitor of platelet-derived growth factor receptors (PDGFRα and PDGFRβ), vascular endothelial growth factor receptors (VEGFR1, VEGFR2, and VEGFR3), stem cell factor receptor (KIT), Fms-like tyrosine kinase-3 (FLT3), colony stimulating factor receptor Type 1 (CSF-1R), and the glial cell-line derived neurotrophic factor receptor (RET). Sunitinib inhibition of the activity of these RTKs has been demonstrated in biochemical and cellular assays, and inhibition of function has been demonstrated in cell proliferation assays. The primary metabolite exhibits similar potency compared to sunitinib in biochemical and cellular assays.
Sunitinib inhibited the phosphorylation of multiple RTKs (PDGFRβ, VEGFR2, KIT) in tumor xenografts expressing RTK targets in vivo and demonstrated inhibition of tumor growth or tumor regression and/or inhibited metastases in some experimental models of cancer. Sunitinib demonstrated the ability to inhibit growth of tumor cells expressing dysregulated target RTKs (PDGFR, RET, or KIT) in vitro and to inhibit PDGFRβ- and VEGFR2-dependent tumor angiogenesis in vivo.
What is the most important information I should know about Sutent?
Sutent can cause serious side effects including:
- Severe liver problems, that can lead to death. Tell your healthcare provider right away if you develop any of the following signs and symptoms of liver problems during treatment with Sutent:
- itching
- yellow eyes or skin
- dark urine
- pain or discomfort in the right upper stomach area
Your healthcare provider should do blood tests to check your liver function before you start taking and during treatment with Sutent. Your healthcare provider may tell you to temporarily or permanently stop taking Sutent if you develop liver problems.
See “What are the possible side effects of Sutent?” for more information about side effects.
What should I tell my healthcare provider before taking Sutent?
Before taking Sutent tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems
- have high blood pressure
- have thyroid problems
- have a history of low blood sugar or diabetes
- have kidney function problems (other than cancer)
- have liver problems
- have any bleeding problem
- plan to have any surgery or dental procedures
- have seizures
- have or have had pain in the mouth, teeth or jaw, swelling or sores inside the mouth, numbness or a feeling of heaviness in the jaw, or loosening of a tooth
- are pregnant or plan to become pregnant. Sutent can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with Sutent.
- You should use effective birth control (contraception) during treatment and for at least 4 weeks after your last dose of Sutent.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with Sutent.
Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment and for 7 weeks after your last dose of Sutent.
Sutent may cause fertility problems in males and females. Tell your healthcare provider if this is a concern for you. - are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with Sutent and for at least 4 weeks (1 month) after the last dose.
Tell all of your healthcare providers and dentists that you are taking Sutent. They should talk to the healthcare provider who prescribed Sutent for you, before you have any surgery, or medical or dental procedure.
Tell your healthcare provider about all the medicines you take, including prescription medicines and over-the-counter medicines, vitamins, and herbal supplements. Using Sutent with certain other medicines can cause serious side effects.
You may have an increased risk of severe jaw bone problems (osteonecrosis) if you take Sutent and a bisphosphonate medicine. Especially tell your healthcare provider if you are taking or have taken an osteoporosis medicine.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Sutent?
- Take Sutent exactly the way your healthcare provider tells you.
- Take Sutent 1 time each day with or without food.
- If you take Sutent for GIST or RCC, you will usually take your medicine for 4 weeks (28 days) and then stop for 2 weeks (14 days). This is 1 cycle of treatment. You will repeat this cycle for as long as your healthcare provider tells you to.
- If you take Sutent for pNET, take it 1 time each day until your healthcare provider tells you to stop.
- Do not drink grapefruit juice or eat grapefruit during your treatment with Sutent. They may cause you to have too much Sutent in your body.
- Your healthcare provider may do blood tests before each cycle of treatment to check you for side effects.
- If you miss a dose of Sutent by less than 12 hours, take the missed dose right away. If you miss a dose of Sutent by more than 12 hours, just take your next dose at your regular time. Do not make up the missed dose. Tell your healthcare provider about any missed dose.
- Call your healthcare provider right away, if you take too much Sutent.
What are the possible side effects of Sutent?
Sutent may cause serious side effects, including:
- See “What is the most important information I should know about Sutent?”
- Heart problems. Heart problems may include heart failure, heart attack and heart muscle problems (cardiomyopathy) that can lead to death. Tell your healthcare provider if you feel very tired, are short of breath, or have swollen feet and ankles. Your healthcare provider may stop your treatment with Sutent if you have signs and symptoms of heart failure.
- Abnormal heart rhythm changes. Changes in the electrical activity of your heart called QT prolongation can cause irregular heart beats that can be life threatening. Your healthcare provider may do electrocardiograms and blood tests (electrolytes) to watch for these problems during your treatment with Sutent. Tell your healthcare provider immediately if you feel dizzy, faint, or have abnormal heartbeats during your treatment with Sutent
- you feel faint or lightheaded, or you pass out
- dizziness
- feel your heart beat is irregular or fast
- High blood pressure. High blood pressure is common with Sutent, and may sometimes be severe. Follow your healthcare provider’s instructions about having your blood pressure checked regularly. Call your healthcare provider if your blood pressure is high, or if you have any of the following signs or symptoms of high blood pressure:
- severe headache
- lightheadedness
- dizziness
- change in visionYour healthcare provider may prescribe medicine for you to treat high blood pressure, if needed. Your healthcare provider may temporarily stop your treatment with Sutent until your high blood pressure is controlled.
- Bleeding problems. Bleeding is common with Sutent, but Sutent can also cause severe bleeding problems that can lead to death. Call your healthcare provider right away if you have any of these symptoms or a serious bleeding problem during treatment with Sutent, including:
- painful, swollen stomach (abdomen)
- vomiting blood
- black, sticky stools
- bloody urine
- headache or change in your mental status
- coughing up bloodYour healthcare provider:
- can tell you about other symptoms to watch for
- may do blood tests if needed and monitor you for bleeding
- Serious stomach and intestinal problems, that can sometimes lead to death. Some people have had tears in their stomach or intestine (perforation), or have developed an abnormal opening between the stomach and intestine (fistula). Get medical help right away if you get stomach-area (abdominal) pain that does not go away or is severe during treatment with Sutent.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells and may lead to death. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
- Thrombotic microangiopathy (TMA) including thrombotic thrombocytopenia purpura (TTP) and hemolytic uremic syndrome (HUS). TMA is a condition that involves injury to the smallest blood vessels, and blood clots that can happen while taking Sutent. TMA is accompanied by a decrease in red cells and cells that are involved with clotting. TMA may harm your body’s organs such as the brain and kidneys, and can sometimes lead to death. Your healthcare provider may tell you to stop taking Sutent if you develop TMA.
- Protein in your urine. Some people who have taken Sutent have developed protein in their urine, and in some cases, kidney problems that can lead to death. Your healthcare provider will check you for this problem. If there is too much protein in your urine, your healthcare provider may tell you to stop taking Sutent.
- Serious skin and mouth reactions. Treatment with Sutent has caused severe skin reactions that can lead to death, including:
- severe rash with blisters or peeling of the skin.
- painful sores or ulcers on the skin, lips or inside the mouth.
- tissue damage (necrotizing fasciitis).If you have any signs or symptoms of severe skin reactions, stop taking Sutent and call your healthcare provider or get medical help right away.
- Thyroid problems. Your healthcare provider may do tests to check your thyroid function during Sutent treatment. Tell your healthcare provider if you have any of the following signs and symptoms during your treatment with Sutent:
- tiredness that gets worse and does not go away
- loss of appetite
- problems with heat
- feeling nervous or agitated, tremors
- sweating
- nausea or vomiting
- diarrhea
- fast heat rate
- weight gain or weight loss
- feeling depressed
- irregular menstrual periods or no menstrual periods
- headache
- hair loss
- Low blood sugar (hypoglycemia). Low blood sugar can happen with Sutent, and may cause you to become unconscious, or you may need to be hospitalized. Low blood sugar with Sutent may be worse in people who have diabetes and take antidiabetic medicines. Your healthcare provider should check your blood sugar levels regularly during treatment with Sutent and may need to adjust the dose of your antidiabetic medicines. Signs and symptoms of low blood sugar may include:
- headache
- drowsiness
- weakness
- dizziness
- confusion
- irritability
- hunger
- fast heart beat
- sweating
- feeling jitteryCall your healthcare provider right away if you have any signs or symptoms of severe low blood sugar during your treatment with Sutent.
- Jaw-bone problems (osteonecrosis). Severe jaw bone problems have happened in some people who take Sutent. Certain risk factors such as taking a bisphosphonate medicine or having dental disease may increase your risk of getting osteonecrosis. Your healthcare provider may tell you to see your dentist before you start taking Sutent. Your healthcare provider may tell you to avoid dental procedures, if possible, during your treatment with Sutent, especially if you are receiving a bisphosphonate medicine into a vein (intravenous).
- Wound healing problems. Wounds may not heal properly during Sutent treatment. Tell your healthcare provider if you have or plan to have any surgery before starting or during treatment with Sutent.
- Your healthcare provider may tell you to temporarily stop taking Sutent if you are planning to have certain types of surgery.
- Your healthcare provider should tell you when you may start taking Sutent again after surgery.
Common side effects of Sutent include:
- tiredness
- weakness
- diarrhea
- pain, swelling or sores inside of your mouth
- nausea
- loss of appetite
- indigestion
- vomiting
- stomach-area (abdominal) pain
- blisters or rash on the palms of your hands and soles of your feet
- high blood pressure
- taste changes
- low platelet counts
The medicine in Sutent is yellow, and it may make your skin look yellow. Your skin and hair may get lighter in color. Sutent may also cause other skin problems including: dryness, thickness or cracking of the skin.
These are not all of the possible side effects of Sutent. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Sutent
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Sutent for a condition for which it was not prescribed. Do not give Sutent to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Sutent that is written for health professionals.
How should I store Sutent?
- Store Sutent at room temperature, between 68°F to 77°F (20°C to 25°C).
Keep Sutent and all medicines out of the reach of children.
What are the ingredients in Sutent?
Active ingredient: sunitinib malate
Inactive ingredients: mannitol, croscarmellose sodium, povidone (K-25), and magnesium stearate.
Orange gelatin capsule shells: titanium dioxide, and red iron oxide.
Caramel gelatin capsule shells: titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide.
Yellow gelatin capsule shells: titanium dioxide and yellow iron oxide.
White printing ink: shellac, propylene glycol, sodium hydroxide, povidone, and titanium dioxide.
Black printing ink: shellac, propylene glycol, potassium hydroxide and black iron oxide.
Label
PRINCIPAL DISPLAY PANEL – 12.5 MG CAPSULE BOTTLE LABEL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
- NDC 0069-0550-38
- Pfizer
- Sutent®
(sunitinib malate) - 12.5 mg*
- Capsules
- 28 Capsules
Rx only


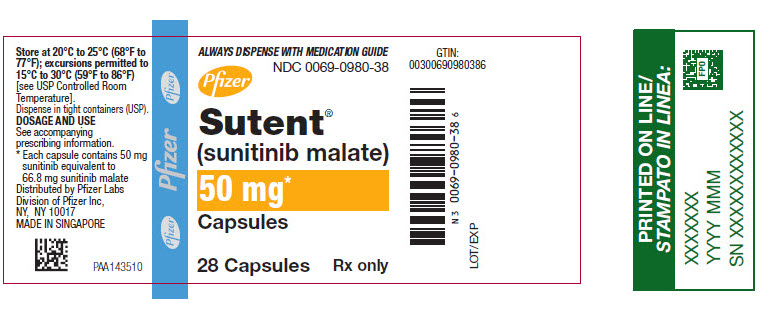
PRINCIPAL DISPLAY PANEL – 50 MG CAPSULE BOTTLE LABEL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
- NDC 0069-0980-38
- Pfizer
- Sutent®
(sunitinib malate) - 50 mg*
- Capsules
- 28 Capsules
Rx only

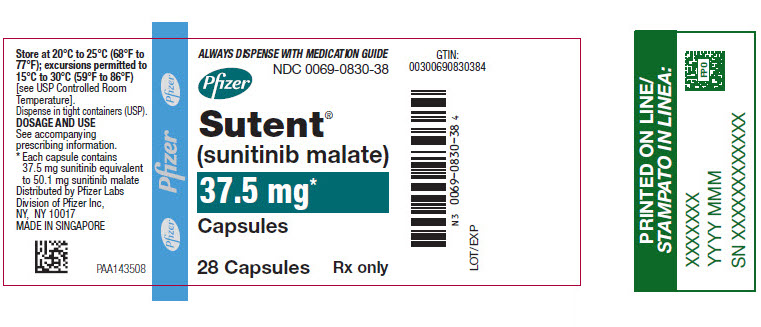
PRINCIPAL DISPLAY PANEL – 37.5 MG CAPSULE BOTTLE LABEL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
- NDC 0069-0830-38
- Pfizer
- Sutent®
(sunitinib malate) - 37.5 mg*
- Capsules
- 28 Capsules
Rx only
SRC: NLM .
