Supprelin LA
Generic name: Histrelin Implant (CPP)
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
Medically reviewed by A Ras MD.
What is Supprelin LA?
Supprelin LA is an implanted gonadotropin releasing hormone (GnRH) medicine used for the treatment of children with central precocious puberty (CPP).
It is not known if Supprelin LA is safe and effective in children under 2 years of age.
Description
SUPPRELIN LA is a sterile, non-biodegradable, diffusion-controlled, hydrogel polymer reservoir containing histrelin acetate, a synthetic nonapeptide analog of the naturally occurring gonadotropin releasing hormone (GnRH) possessing a greater potency than the natural sequence hormone. SUPPRELIN LA is designed to deliver approximately 65 mcg histrelin acetate per day over 12 months.
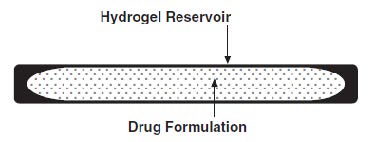
The SUPPRELIN LA implant looks like a small thin flexible tube and consists of a 50-mg histrelin acetate drug core inside a 3.5 cm by 3 mm, cylindrical, hydrogel polymer reservoir (Figure 1). The implant may appear partially to completely full with variation in color from off-white to light brown. The color may be uneven within the core.
Figure 1. SUPPRELIN LA Implant Diagram (not to scale)
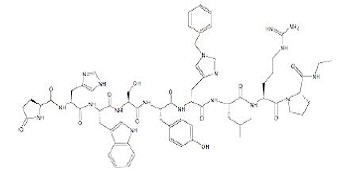
The chemical name of histrelin acetate is: L-Pyroglutamyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-N-benzyl-D-histidyl-L-leucyl-L-arginyl-L-proline N-ethylamide, acetate salt.
The molecular formula for histrelin acetate is C66H86N18O12 x 2 CH3COOH and its molecular weight is 1443.70 (or 1323.52 as free base). Histrelin is also chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide diacetate. The chemical structure of the free base (histrelin) is represented below in Figure 2.
Figure 2. Structure of Histrelin
The drug core also contains the inactive ingredient stearic acid NF. The hydrogel polymer reservoir is a hydrophilic cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. Each implant is packaged hydrated in a glass vial containing 2 mL of sterile 1.8% sodium chloride solution, so that it is primed for immediate release of the drug upon insertion.
A single use, sterile, Insertion Tool is provided along with the implant that can be used for the placement of the SUPPRELIN LA implant into the subcutaneous tissue of the inner aspect of the upper arm. The Insertion Tool is enclosed in a sterile bag and is provided separately from the implant in the Implantation Kit [see Recommended Procedure for Implant Insertion and Removal
Mechanism of Action
SUPPRELIN LA is a GnRH agonist and an inhibitor of gonadotropin secretion when given continuously. It delivers approximately 65 mcg histrelin acetate per day. Both animal and human studies indicate that following an initial stimulatory phase, chronic, subcutaneous administration of histrelin acetate desensitizes responsiveness of the pituitary gonadotropin which, in turn causes a reduction in ovarian and testicular steroidogenesis.
In humans, administration of histrelin acetate results in an initial increase in circulating levels of LH and FSH, leading to a transient increase in concentration of gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females).
However, continuous administration of histrelin acetate causes a reversible down-regulation of the GnRH receptors in the pituitary gland and desensitization of the pituitary gonadotropes. These inhibitory effects result in decreased levels of LH and FSH.
What is the most important information I should know about Supprelin LA?
- In the first week of treatment, Supprelin LA can cause an increase in some hormones. During this time you may notice more signs of puberty in your child, including light vaginal bleeding and breast enlargement in girls.Within 4 weeks of treatment, you should see signs in your child that puberty is stopping.
- Some people who had Supprelin LA placed in their arm have had the implant come through the skin (extrusion). Call your child’s doctor right away if the Supprelin LA implant comes through the skin.
- Some people taking GnRH agonists like Supprelin LA have had new or worsening mental (psychiatric) problems. Mental (psychiatric) problems may include emotional symptoms such as:
- crying
- irritability
- restlessness (impatience)
- anger
- acting aggressive
Call your child’s doctor right away if your child has any new or worsening mental symptoms or problems while taking Supprelin LA.
- Some people taking GnRH agonists like Supprelin LA have had seizures. The risk of seizures may be higher in people who:
- have a history of seizures
- have a history of epilepsy
- have a history of brain or brain vessel (cerebrovascular) problems or tumors
- are taking a medicine that has been connected to seizures such as bupropion or selective serotonin reuptake inhibitors (SSRIs)
Seizures have also happened in people who have not had any of these problems.
Call your child’s doctor right away if your child has a seizure while taking Supprelin LA.
Who should not take Supprelin LA?
Supprelin LA should not be taken if your child is:
- allergic to gonadotropin releasing hormone (GnRH), GnRH agonist medicines, or any ingredients in the Supprelin LA implant. See the end of this Medication Guide for a complete list of ingredients in Supprelin LA.
- pregnant or becomes pregnant. Supprelin LA can cause birth defects or loss of the baby.If your child becomes pregnant call your doctor.
What should I tell my healthcare provider before using Supprelin LA?
Before your child receives Supprelin LA, tell the doctor about all of your child’s medical conditions, including if they:
- have a history of mental (psychiatric) problems.
- have a history of seizures.
- have a history of epilepsy.
- have a history of brain or brain vessel (cerebrovascular) problems or tumors.
- are taking a medicine that has been connected to seizures such as bupropion or selective serotonin reuptake inhibitors (SSRIs).
Tell your doctor about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Supprelin LA?
- Your child’s doctor should do tests to make sure your child has CPP before treating with Supprelin LA.
- Supprelin LA lasts for 12 months. One implant will give the medicine for 12 months. After 12 months, the Supprelin LA implant must be removed. The doctor may place a new Supprelin LA implant at this time to continue treatment.
- Supprelin LA is placed under the skin of the inside of the upper arm. The doctor will numb the arm of your child, make a small cut, and then place the Supprelin LA implant under the skin. The cut may be closed with stitches or surgical strips and covered with a pressure bandage.
- Your child should keep the arm clean and dry and should not swim or bathe for 24 hours after receiving the Supprelin LA implant. The bandage can be removed after 24 hours. Do not remove any surgical strips. Surgical strips will fall off on their own in a few days.
- Your child should avoid heavy play or exercise that uses the arm where the implant was placed for 7 days. After the cut has healed, your child can go back to his or her normal activities. The doctor will give you complete instructions.
- Keep all scheduled visits to the doctor. The doctor will do regular exams and blood tests to check for signs of puberty.
- Sometimes the doctor will have to do special tests, such as an ultrasound, computed tomography (CT) scan, or magnetic resonance imaging (MRI) if the Supprelin LA implant is hard to find under your child’s skin.
What are the possible side effects of Supprelin LA?
Supprelin LA may cause serious side effects. See “What is the most important information I should know about Supprelin LA?”
The most common side effect of Supprelin LA includes skin reactions at the place where the implant is inserted. These reactions may include pain, redness, bruising, soreness, and swelling in and around the implant site. Call your child’s doctor if your child has bleeding, redness, or severe pain where the implant was inserted.
These are not all the possible side effects of Supprelin LA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What are the ingredients in Supprelin LA?
Active ingredient: histrelin acetate
Inactive ingredients: stearic acid NF, hydrogel polymer reservoir composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100
Label
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – Vial Label
PRINCIPAL DISPLAY PANEL