Harvoni
Generic name: ledipasvir and sofosbuvir
Drug class: Antiviral combinations
Medically reviewed by A Ras MD.
What is Harvoni?
Harvoni is a prescription medicine used to treat adults and children 3 years of age and older with chronic (lasting a long time) hepatitis C virus (HCV), genotype 1, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis, genotype 1 infection with advanced cirrhosis (decompensated) in combination with ribavirin, genotype 1 or 4 infection without cirrhosis or with compensated cirrhosis who have had a liver transplant, in combination with ribavirin.
It is not known if Harvoni is safe and effective in children with HCV under 3 years of age.
Description
HARVONI is a fixed-dose combination tablet containing ledipasvir and sofosbuvir for oral administration. Ledipasvir is an HCV NS5A inhibitor and sofosbuvir is a nucleotide analog inhibitor of HCV NS5B polymerase.
Each tablet contains 90 mg ledipasvir and 400 mg sofosbuvir. The tablets include the following inactive ingredients: colloidal silicon dioxide, copovidone, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing the following inactive ingredients: polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Ledipasvir: The IUPAC name for ledipasvir is Methyl [(2S)-1-{(6S)-6-[5-(9,9-difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carbamate.
It has a molecular formula of C49H54F2N8O6 and a molecular weight of 889.00. It has the following structural formula:
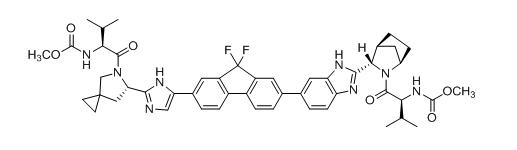
Ledipasvir is practically insoluble (less than 0.1 mg/mL) across the pH range of 3.0–7.5 and is slightly soluble below pH 2.3 (1.1 mg/mL).
Sofosbuvir: The IUPAC name for sofosbuvir is (S)-Isopropyl 2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)-(phenoxy)phosphorylamino)propanoate. It has a molecular formula of C22H29FN3O9P and a molecular weight of 529.45. It has the following structural formula:
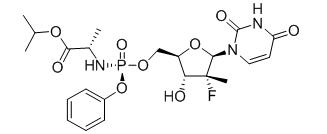
Sofosbuvir is a white to off-white crystalline solid with a solubility of at least 2 mg/mL across the pH range of 2–7.7 at 37°C and is slightly soluble in water.
Mechanism of Action
HARVONI is a fixed-dose combination of ledipasvir and sofosbuvir which are direct-acting antiviral agents against the hepatitis C virus
What is the most important information I should know about Harvoni?
Important: If you take Harvoni with ribavirin, you should also read the Medication Guide for ribavirin.
Harvoni can cause serious side effects, including,
Hepatitis B virus reactivation: Before starting treatment with Harvoni, your healthcare provider will do blood tests to check for hepatitis B virus infection. If you have ever had hepatitis B virus infection, the hepatitis B virus could become active again during or after treatment of hepatitis C virus with Harvoni. Hepatitis B virus becoming active again (called reactivation) may cause serious liver problems including liver failure and death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop taking Harvoni.
For more information about side effects, see the section “What are the possible side effects of Harvoni?”
What should I tell my healthcare provider before taking Harvoni?
Before taking Harvoni, tell your healthcare provider about all of your medical conditions, including if you:
- have ever had hepatitis B virus infection
- have liver problems other than hepatitis C infection
- have had a liver transplant
- have kidney problems or you are on dialysis
- have HIV infection
- are pregnant or plan to become pregnant. It is not known if Harvoni will harm your unborn baby.
- Males and females who take Harvoni in combination with ribavirin should also read the ribavirin Medication Guide for important pregnancy, contraception, and infertility information.
- are breastfeeding or plan to breastfeed. It is not known if Harvoni passes into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby during treatment with Harvoni.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Harvoni and other medicines may affect each other. This can cause you to have too much or not enough Harvoni or other medicines in your body. This may affect the way Harvoni or your other medicines work, or may cause side effects. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Harvoni.
Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Harvoni with other medicines.
How should I take Harvoni?
- Take Harvoni exactly as your healthcare provider tells you to take it. Do not change your dose unless your healthcare provider tells you to.
- Do not stop taking Harvoni without first talking with your healthcare provider.
- Take Harvoni tablets or oral pellets by mouth, with or without food.
- It is important that you do not miss or skip doses of Harvoni during treatment.
- For adults the usual dose of Harvoni is one 90/400 mg tablet each day.
- For children 3 years of age and older your healthcare provider will prescribe the right dose of Harvoni tablets or oral pellets based on your child’s body weight.
- Tell your healthcare provider if your child has problems with swallowing tablets.
- If your healthcare provider prescribes Harvoni oral pellets for your child, see below for “How should I give Harvoni oral pellets to my child?”
- Do not miss a dose of Harvoni. Missing a dose lowers the amount of medicine in your blood. Refill your Harvoni prescription before you run out of medicine.
If you take too much Harvoni, call your healthcare provider or go to the nearest hospital emergency room right away.
How should I give Harvoni oral pellets to my child?
- Administer Harvoni oral pellets exactly as instructed by your healthcare provider.
- Do not open the packet until ready to use.
- Hold the Harvoni pellets packet with the cut line on top.
- Shake the Harvoni pellets packet gently to settle the pellets.
- Tear or cut the Harvoni packet along the cut line.
- Harvoni oral pellets can be taken right in the mouth without chewing, or with food.
- If Harvoni pellets are taken with food, sprinkle the pellets on one or more spoonfuls of non-acidic soft food at or below room temperature. Examples of non-acidic foods include pudding, chocolate syrup, mashed potato, and ice cream. Take Harvoni pellets within 30 minutes of gently mixing with food and swallow the entire contents without chewing to avoid a bitter taste.
- Do not store any leftover Harvoni mixture (oral pellets mixed with food) for use at a later time. Throw away any unused portion.
What are the possible side effects of Harvoni?
Harvoni can cause serious side effects, including:
- Hepatitis B virus reactivation. See “What is the most important information I should know about Harvoni?”
- Slow heart rate (bradycardia). Harvoni treatment may result in slowing of the heart rate along with other symptoms when taken with amiodarone (Cordarone, Nexterone, Pacerone), a medicine used to treat certain heart problems. In some cases bradycardia has led to death or the need for a heart pacemaker when amiodarone is taken with Harvoni. Get medical help right away if you take amiodarone with Harvoni and get any of the following symptoms:
- fainting or near-fainting
- dizziness or lightheadedness
- not feeling well
- weakness
- extreme tiredness
- shortness of breath
- chest pains
- confusion
- memory problems
The most common side effects of Harvoni include:
- tiredness
- headache
- weakness
These are not all the possible side effects of Harvoni. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Harvoni
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Harvoni for a condition for which it was not prescribed. Do not give Harvoni to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Harvoni that is written for health professionals.
How should I store Harvoni?
- Store Harvoni tablets or pellets below 86°F (30°C).
- Keep Harvoni tablets in the original container.
- Do not use Harvoni tablets if the seal over the bottle opening is broken or missing.
- Do not use Harvoni pellets if the carton tamper-evident seal, or the pellets packet seal, is broken or damaged.
Keep Harvoni and all medicines out of the reach of children.
What are the ingredients in Harvoni?
Active ingredients: ledipasvir and sofosbuvir
Inactive ingredients
Tablets: colloidal silicon dioxide, copovidone, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose.
Tablet film coat for 90/400mg: FD&C yellow #6/sunset yellow FCF aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Tablet film coat for 45/200mg: polyethylene glycol, polyvinyl alcohol partially hydrolyzed, talc, and titanium dioxide.
Oral Pellets: amino-methacrylate copolymer, colloidal silicon dioxide, copovidone, croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, talc, and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 28 TABLET BOTTLE LABEL
- 61958-1802-628 tablets
- Harvoni™(ledipasvir, sofosbuvir) Tablets
90 mg / 400 mg
- GILEAD ACCESS PROGRAM
- Note to pharmacist:Do not cover ALERT box with pharmacy label.
- ALERT: Find out about medicines thatshould NOT be taken with Harvoni™


SRC: NLM .