Inqovi
Generic name: cedazuridine and decitabine
Drug class: Antineoplastic combinations
Medically reviewed by A Ras MD.
What is Inqovi?
Description
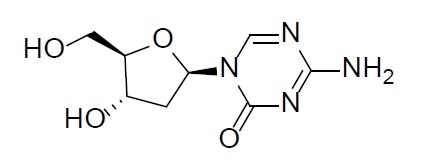
Decitabine
Decitabine is a nucleoside metabolic inhibitor. Decitabine is a white to off-white solid with the molecular formula of C8H12N4O4 and a molecular weight of 228.21 daltons. Its international union of pure and applied chemistry (IUPAC) chemical name is 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2(1H)-one and it has the following structural formula:
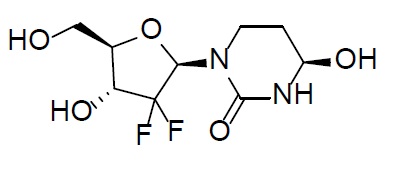
Cedazuridine
Cedazuridine is a cytidine deaminase inhibitor. Cedazuridine is a white to off-white solid with the molecular formula of C9H14F2N2O5 and a molecular weight of 268.21 daltons. Its IUPAC chemical name is (4R)-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one and it has the following structural formula:
INQOVI
INQOVI (decitabine and cedazuridine) tablets, for oral use contain 35 mg decitabine and 100 mg cedazuridine. The tablets are biconvex, oval-shaped, film-coated, red and debossed with “H35” on one side. Each film-coated tablet contains the following inactive ingredients: lactose monohydrate, hypromellose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate. The film coating material contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
Mechanism of Action
Decitabine is a nucleoside metabolic inhibitor that is believed to exert its effects after phosphorylation and direct incorporation into DNA and inhibition of DNA methyltransferase, causing hypomethylation of DNA and cellular differentiation and/or apoptosis. Decitabine inhibits DNA methylation in vitro, which is achieved at concentrations that do not cause major suppression of DNA synthesis. Decitabine-induced hypomethylation in cancer cells may restore normal function to genes that are critical for the control of cellular differentiation and proliferation. In rapidly dividing cells, the cytotoxicity of decitabine may also be attributed to the formation of covalent adducts between DNA methyltransferase and decitabine incorporated into DNA. Non-proliferating cells are relatively insensitive to decitabine.
Cytidine deaminase (CDA) is an enzyme that catalyzes the degradation of cytidine, including the cytidine analog decitabine. High levels of CDA in the gastrointestinal tract and liver degrade decitabine and limit its oral bioavailability. Cedazuridine is a CDA inhibitor. Administration of cedazuridine with decitabine increases systemic exposure of decitabine.
What should I tell my healthcare provider before taking Inqovi?
Before taking Inqovi, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems
- have liver problems
- are pregnant or plan to become pregnant. Inqovi can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Inqovi.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before you start treatment with Inqovi.
- You should use effective birth control during treatment with Inqovi and for at least 6 months after your last dose of Inqovi.
Males with female partners who are able to become pregnant should use effective birth control during treatment with Inqovi and for 3 months after the last dose. Talk to your healthcare provider if you have questions about birth control options that are right for you.
- are breastfeeding or plan to breastfeed. It is not known if Inqovi passes into breast milk. Do not breastfeed during treatment with Inqovi and for at least 2 weeks after your last dose of Inqovi.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Inqovi?
- Take Inqovi exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking Inqovi unless your healthcare provider tells you to.
- Your healthcare provider may tell you to decrease your dose, temporarily stop, or completely stop taking Inqovi if you get certain side effects.
- Take Inqovi one time a day at about the same time each day.
- Take Inqovi on an empty stomach. Do not eat for at least 2 hours before and 2 hours after taking Inqovi.
- Swallow Inqovi tablets whole. Do not cut, crush, or chew the tablet.
- If you miss a dose of Inqovi, take your dose as soon as possible if it is within 12 hours of your usual time. Then, continue taking Inqovi at your scheduled time. If you missed a dose by more than 12 hours, do not take additional doses to make up for the missed dose. Take your next scheduled dose on the following day at your usual time.
- If you vomit after taking a dose of Inqovi, do not take an additional dose. Take your next scheduled dose at your usual time.
What are the possible side effects of Inqovi?
Inqovi may cause serious side effects, including:
- Low blood cell counts. Low blood counts (white blood cells, platelets, and red blood cells) are common with Inqovi but can also be serious and lead to infections that may be life-threatening. If your blood cell counts are too low, your healthcare provider may need to delay treatment with Inqovi, lower your dose of Inqovi, or in some cases give you a medicine to help treat low blood cell counts. Your healthcare provider may need to give you antibiotic medicines to prevent or treat infections or fever while your blood cell counts are low. Your healthcare provider will check your blood cell counts before you start treatment and regularly during treatment with Inqovi. Call your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with Inqovi:
- fever
- chills
- body aches
- bruising more easily than usual
The most common side effects of Inqovi include:
- low white blood cell count (leukopenia)
- low platelets in your blood (thrombocytopenia)
- low white blood cell count (neutropenia)
- low red blood cell count (anemia)
- tiredness
- constipation
- bleeding
- muscle pain
- pain or sores in your mouth or throat
- joint pain
- nausea
- shortness of breath
- diarrhea
- rash
- dizziness
- fever with low white blood cell count (febrile neutropenia)
- swelling of arms or legs
- headache
- cough
- decreased appetite
- upper respiratory tract infection
- pneumonia
- changes in liver function tests
Inqovi may affect fertility in men. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Inqovi. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Inqovi
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Inqovi for a condition for which it was not prescribed. Do not give Inqovi to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Inqovi that is written for health professionals.
How should I store Inqovi?
- Store Inqovi at room temperature between 68°F and 77°F (20°C and 25°C).
- Do not store Inqovi outside of the original blisters.
- Talk to your healthcare provider about how to safely throw away (dispose of) Inqovi.
Keep Inqovi and all medicines out of the reach of children.
What are the ingredients in Inqovi?
Active ingredients: decitabine and cedazuridine
Inactive ingredients: lactose monohydrate, hypromellose, croscarmellose sodium, colloidal silicon dioxide and magnesium stearate. The film coating material contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
Label
PRINCIPAL DISPLAY PANEL – 35 MG/100 MG TABLET CARTON
- NDC 64842-0727-9
Rx Only - INQOVI®
(decitabine and cedazuridine) tablets - 35 mg/100 mg per tablet
- CAUTION: Hazardous Agent
- One Blister card
containing 5 tablets - TAIHO
ONCOLOGY, INC.


SRC: NLM .