Lenvima
Generic name: lenvatinib
Drug classes: Multikinase inhibitors, VEGF/VEGFR inhibitors
Medically reviewed by A Ras MD.
What is Lenvima?
Lenvima is a prescription medicine that is used to treat people with certain kinds of cancer. Lenvima is used by itself to treat differentiated thyroid cancer (DTC), a type of thyroid cancer that can no longer be treated with radioactive iodine and is progressing. Lenvima is used to treat adults with a type of kidney cancer called advanced renal cell carcinoma (RCC) along with the medicine pembrolizumab as your first treatment when your kidney cancer has spread or cannot be removed by surgery.
It is also used along with the medicine everolimus after one course of treatment with another anti-cancer medicine.
Lenvima is used by itself as the first treatment for a type of liver cancer called hepatocellular carcinoma (HCC) when it cannot be removed by surgery.
Lenvima is used along with another medicine called pembrolizumab to treat endometrial carcinoma, a type of uterine cancer:
- when your tumor is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), and
- you have received anti-cancer treatment, and it is no longer working, and
- your cancer cannot be cured by surgery or radiation (advanced endometrial carcinoma).
It is not known if Lenvima is safe and effective in children.
Description
LENVIMA, a kinase inhibitor, is the mesylate salt of lenvatinib. Its chemical name is 4-[3-chloro-4-(N’-cyclopropylureido)phenoxy]-7-methoxyquinoline-6-carboxamide methanesulfonate. The molecular formula is C21H19ClN4O4 • CH4O3S, and the molecular weight of the mesylate salt is 522.96. The chemical structure of lenvatinib mesylate is:
![The chemical structure of lenvatinib mesylate is LENVIMA, a kinase inhibitor, is the mesylate salt of lenvatinib. Its chemical name is 4-[3-chloro-4-(N’-cyclopropylureido)phenoxy]-7-methoxyquinoline-6 carboxamide methanesulfonate. The molecular formula is C21H19ClN4O4 • CH4O3S, and the molecular weight of the mesylate salt is 522.96.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=lenvima-01.jpg&setid=f4bedd21-efde-44c6-9d9c-b48b78d7ed1e)
Lenvatinib mesylate is a white to pale reddish yellow powder. It is slightly soluble in water and practically insoluble in ethanol (dehydrated). The dissociation constant (pKa value) of lenvatinib mesylate is 5.05 at 25°C. The partition coefficient (log P value) is 3.3.
LENVIMA capsules for oral administration contain 4 mg or 10 mg of lenvatinib, equivalent to 4.90 mg or 12.25 mg of lenvatinib mesylate, respectively. The inactive ingredients are: calcium carbonate, hydroxypropyl cellulose, low-substituted hydroxypropyl cellulose, mannitol, microcrystalline cellulose, and talc.
In addition, the capsule shell contains ferric oxide red, ferric oxide yellow, hypromellose, and titanium dioxide. The printing ink contains black iron oxide, potassium hydroxide, propylene glycol, and shellac.
Mechanism of Action
Lenvatinib is a kinase inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). Lenvatinib inhibits other kinases that have been implicated in pathogenic angiogenesis, tumor growth, and cancer progression in addition to their normal cellular functions, including fibroblast growth factor (FGF) receptors FGFR1, 2, 3, and 4; platelet derived growth factor receptor alpha (PDGFRα), KIT, and RET. Lenvatinib also exhibited antiproliferative activity in hepatocellular carcinoma cell lines dependent on activated FGFR signaling with a concurrent inhibition of FGF-receptor substrate 2α (FRS2α) phosphorylation.
In syngeneic mouse tumor models, lenvatinib decreased tumor-associated macrophages, increased activated cytotoxic T cells, and demonstrated greater antitumor activity in combination with an anti-PD-1 monoclonal antibody compared to either treatment alone.
The combination of lenvatinib and everolimus showed increased antiangiogenic and antitumor activity as demonstrated by decreases in human endothelial cell proliferation, tube formation, and VEGF signaling in vitro, and by decreases in tumor volume in mouse xenograft models of human renal cell cancer that were greater than those with either drug alone.
What should I tell my healthcare provider before taking Lenvima?
Before you take Lenvima, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood pressure
- have heart problems
- have a history of blood clots in your arteries (type of blood vessel), including stroke, heart attack, or change in vision
- have or have had liver or kidney problems
- have a history of a tear (perforation) in your stomach or intestine, or an abnormal connection between two or more body parts (fistula)
- have headaches, seizures, or vision problems
- have any bleeding problems
- plan to have surgery or have had a recent surgery. You should stop taking Lenvima at least 1 week before planned surgery. See “What are the possible side effects of Lenvima?”
- are pregnant or plan to become pregnant. Lenvima can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with Lenvima.
- You should use an effective method of birth control during treatment with Lenvima and for at least 30 days after the last dose of Lenvima. Talk with your healthcare provider about birth control methods you can use during this time. Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with Lenvima.
- are breastfeeding or plan to breastfeed. It is not known if Lenvima passes into your breast milk. Do not breastfeed during treatment with Lenvima and for at least 1 week after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you are taking, or have taken, an osteoporosis medicine.
Know the medicines you take. Keep a list of your medicines to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Lenvima?
- Take Lenvima exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much Lenvima to take and when to take it. Your healthcare provider may change your dose during treatment, stop treatment for some time, or completely stop treatment with Lenvima if you have side effects.
- Take Lenvima 1 time each day at the same time, with or without food.
- If you miss a dose of Lenvima, take it as soon as you remember. If your next dose is due within 12 hours, skip the missed dose and take the next dose at your regular time.
- If you cannot swallow Lenvima capsules whole:
- Use a medicine cup to measure about one tablespoon of water or apple juice and place into a small glass.
- Place the Lenvima capsules into the small glass without breaking or crushing them.
- Leave the capsules in the liquid for at least 10 minutes.
- Stir the contents of the glass for at least 3 minutes.
- Drink the mixture. After drinking, rinse the glass with a small amount of additional water or apple juice and swallow the liquid.
- If you take too much Lenvima, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Lenvima?
Lenvima may cause serious side effects, including:
- high blood pressure (hypertension). High blood pressure is a common side effect of Lenvima and can be serious. Your blood pressure should be well controlled before you start taking Lenvima. Your healthcare provider should check your blood pressure regularly during treatment with Lenvima. If you develop blood pressure problems, your healthcare provider may prescribe medicine to treat your high blood pressure.
- heart problems. Lenvima can cause serious heart problems that may lead to death. Call your healthcare provider right away if you get symptoms of heart problems, such as shortness of breath or swelling of your ankles.
- problem with blood clots in your blood vessels (arteries). Get emergency medical help right away if you get any of the following symptoms:
- severe chest pain or pressure
- trouble talking
- pain in your arms, back, neck or jaw
- sudden severe headache
- shortness of breath
- sudden vision changes
- numbness or weakness on one side of your body
- liver problems. Lenvima may cause liver problems that may lead to liver failure and death. Your healthcare provider will check your liver function before and during treatment with Lenvima. Tell your healthcare provider right away if you have any of the following symptoms:
- your skin or the white part of your eyes turns yellow (jaundice)
- dark “tea colored” urine
- light-colored bowel movements (stools)
- feeling drowsy, confused or loss of consciousness
- kidney problems. Kidney failure, which can lead to death, has happened with Lenvima treatment. Your healthcare provider should do regular blood tests to check your kidneys.
- increased protein in your urine (proteinuria). Proteinuria is a common side effect of Lenvima and can be serious. Your healthcare provider should check your urine for protein before and during your treatment with Lenvima.
- diarrhea. Diarrhea is a common side effect of Lenvima and can be serious. If you get diarrhea, ask your healthcare provider about what medicines you can take to treat your diarrhea. It is important to drink more water when you get diarrhea. Tell your healthcare provider or go to the emergency room, if you are unable to drink enough liquids and your diarrhea is not able to be controlled.
- an opening in the wall of your stomach or intestines (perforation) or an abnormal connection between two or more body parts (fistula). Get emergency medical help right away if you have severe stomach (abdomen) pain.
- changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life threatening. Your healthcare provider will do blood tests before and during your treatment with Lenvima to check the levels of potassium, magnesium, and calcium in your blood, and may check the electrical activity of your heart with an ECG.
- low levels of blood calcium (hypocalcemia). Your healthcare provider will check your blood calcium levels during treatment with Lenvima and may tell you to take a calcium supplement if your calcium levels are low.
- a condition called Reversible Posterior Leukoencephalopathy Syndrome (RPLS). Call your healthcare provider right away if you get: severe headache, seizures, weakness, confusion, or blindness or change in vision.
- bleeding. Lenvima may cause serious bleeding problems that may lead to death. Tell your healthcare provider if you have any signs or symptoms of bleeding during treatment with Lenvima, including:
- severe and persistent nose bleeds
- blood in your urine
- vomiting blood
- coughing up blood or blood clots
- red or black (looks like tar) stools
- heavy or new onset vaginal bleeding
- change in thyroid hormone levels. Your healthcare provider should check your thyroid hormone levels before starting and every month during treatment with Lenvima.
- wound healing problems. Wound healing problems have happened in some people who take Lenvima. Tell your healthcare provider if you plan to have any surgery before or during treatment with Lenvima.
- You should stop taking Lenvima at least 1 week before planned surgery.
- Your healthcare provider should tell you when you may start taking Lenvima again after surgery.
- severe jaw bone problems (osteonecrosis). Severe jaw bone problems have happened in some people who take Lenvima. Certain risk factors such as taking a bisphosphonate medicine or the medicine denosumab, having dental disease, or an invasive dental procedure may increase your risk of getting jaw bone problems. Your healthcare provider should examine your mouth before you start and during treatment with Lenvima. Tell your dentist that you are taking Lenvima. It is important for you to practice good mouth care during treatment with Lenvima. Tell your healthcare provider right away if you get signs or symptoms of jaw bone problems during treatment with Lenvima, including jaw pain, toothache, or sores on your gums. Tell your healthcare provider if you plan to have any dental procedures before or during treatment with Lenvima. You should avoid having invasive dental procedures if possible, during treatment with Lenvima. Stopping your bisphosphonate medicine before an invasive dental procedure may help decrease your risk of getting these jaw problems.
- You should stop taking Lenvima at least 1 week before planned dental surgery or invasive dental procedures.
- Your healthcare provider should tell you when you may start taking Lenvima again after dental procedures.
The most common side effects of Lenvima in people treated for thyroid cancer include:
- tiredness
- headache
- joint and muscle pain
- vomiting
- decreased appetite
- rash, redness, itching, or peeling of your skin on your hands and feet
- weight loss
- stomach (abdomen) pain
- nausea
- hoarseness
- mouth sores
The most common side effects of Lenvima when given with everolimus include:
- tiredness
- cough
- joint and muscle pain
- stomach (abdomen) pain
- decreased appetite
- trouble breathing
- vomiting
- rash
- nausea
- weight loss
- mouth sores
- bleeding
- swelling in your arms and legs
The most common side effects of Lenvima in people treated for liver cancer include:
- tiredness
- rash, redness, itching, or peeling of your skin on your hands and feet
- decreased appetite
- hoarseness
- joint and muscle pain
- bleeding
- weight loss
- change in thyroid hormone levels
- stomach (abdomen) pain
- nausea
The most common side effects of Lenvima when given with pembrolizumab include:
- decrease in thyroid hormone level
- weight loss
- increased blood pressure
- stomach-area (abdomen) pain
- tiredness
- urinary tract infection
- diarrhea
- protein in your urine
- joint and muscle pain
- constipation
- nausea
- headache
- decreased appetite
- bleeding
- vomiting
- rash, redness, itching, or peeling of your skin on your hands and feet
- mouth sores
- hoarseness
- rash
Lenvima may cause fertility problems in males and females. Talk to your healthcare provider if this is a concern for you.
Your healthcare provider may need to reduce your dose of Lenvima, or delay or completely stop treatment, if you have certain side effects.
These are not all the possible side effects of Lenvima.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Lenvima
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Lenvima for a condition for which it was not prescribed. Do not give Lenvima to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Lenvima that is written for health professionals.
How should I store Lenvima?
- Store Lenvima at room temperature, between 68°F to 77°F (20°C to 25°C).
Keep Lenvima and all medicines out of the reach of children.
What are the ingredients in Lenvima?
Active ingredient: lenvatinib
Inactive ingredients: calcium carbonate, hydroxypropyl cellulose, low-substituted hydroxypropylcellulose, mannitol, microcrystalline cellulose, and talc.
The capsule shell contains: hypromellose, titanium dioxide, ferric oxide yellow, and ferric oxide red. The printing ink contains black iron oxide, potassium hydroxide, propylene glycol and shellac.
Label
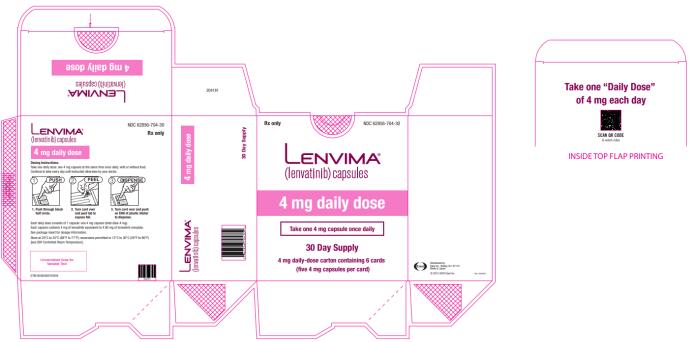
PRINCIPAL DISPLAY PANEL
NDC 62856-704-30
Lenvima
(lenvatinib) capsules
4 mg daily dose

PRINCIPAL DISPLAY PANEL
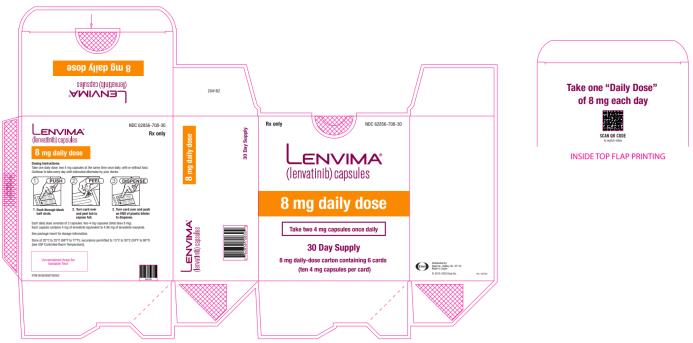
NDC 62856-708-05
Lenvima
(lenvatinib) capsules
8 mg daily dose
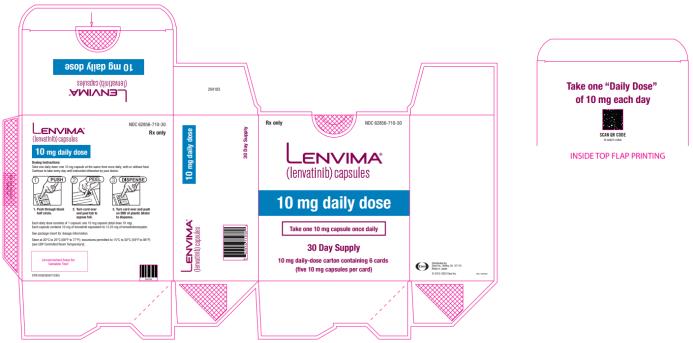
PRINCIPAL DISPLAY PANEL
NDC 62856-710-30
Lenvima
(lenvatinib) capsules
10 mg daily dose
30 day supply
10 mg daily- dose carton containing 6 cards
SRC: NLM .