Lucemyra
Generic name: lofexidine
Drug class: Antiadrenergic agents, centrally acting
Medically reviewed by A Ras MD.
What is Lucemyra?
Lucemyra is a non-opioid prescription medicine used in adults to help with the symptoms of opioid withdrawal that may happen when you stop taking an opioid suddenly.
Lucemyra will not completely prevent the symptoms of opioid withdrawal, which may include feeling sick, stomach cramps, muscle spasms or twitching, feeling of cold, heart pounding, muscular tension, aches and pains, yawning, runny eyes and sleep problems (insomnia).
Lucemyra is not a treatment for opioid use disorder. If you have been diagnosed with opioid use disorder (opioid addiction), your healthcare provider may prescribe Lucemyra as part of a complete treatment program for your opioid use disorder (opioid addiction).
It is not known if Lucemyra is safe and effective in children.
Description
LUCEMYRA tablets contain lofexidine, a central alpha-2 adrenergic agonist, as the hydrochloride salt. Lofexidine hydrochloride is chemically designated as 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5 dihydro-1H– imidazole monohydrochloride with a molecular formula of C11H12Cl2N2O∙HCl. Its molecular weight is 295.6 g/mole and its structural formula is:
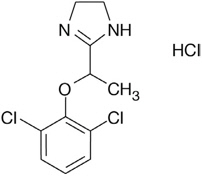
Lofexidine hydrochloride is a white to off-white crystalline powder freely soluble in water, methanol, and ethanol. It is slightly soluble in chloroform and practically insoluble in n-hexane and benzene.
LUCEMYRA is available as round, convex-shaped, peach-colored, film-coated tablets for oral administration. Each tablet contains 0.18 lofexidine, equivalent to 0.2 mg of lofexidine hydrochloride, and the following inactive ingredients: 92.6 mg lactose, 12.3 mg citric acid, 1.1 mg povidone, 5.7 mg microcrystalline cellulose, 1.4 mg calcium stearate, 0.7 mg sodium lauryl sulphate, and Opadry OY S 9480 (contains indigo carmine and sunset yellow).
Mechanism of Action
Lofexidine is a central alpha-2 adrenergic agonist that binds to receptors on adrenergic neurons. This reduces the release of norepinephrine and decreases sympathetic tone.
What is the most important information I should know about Lucemyra?
Lucemyra can cause serious side effects, including low blood pressure (hypotension), slow heart rate (bradycardia), and fainting.
If you get any of the following signs or symptoms, tell your healthcare provider right away:
- low blood pressure
- slow heartbeat
- dizziness
- lightheadedness
- feeling faint at rest or when standing up
If you take Lucemyra at home and have any of these signs and symptoms, do not take your next dose of Lucemyra until you have talked to your healthcare provider. You should avoid becoming dehydrated or overheated during treatment with Lucemyra, which may increase your risk of low blood pressure and fainting. You should also be careful not to stand up too suddenly from lying down or sitting.
When your treatment is complete you will need to stop taking Lucemyra gradually or your blood pressure could increase. For more information about side effects, see “What are the possible side effects of Lucemyra?”
Increased risk of opioid overdose. After a period of time of not using opioid drugs, you can become more sensitive to the effects of opioids if you start using opioids again. This may increase your risk of overdose and death.
What should I tell my healthcare provider before taking Lucemyra?
Before taking Lucemyra, tell your healthcare provider about all of your medical conditions, including if you:
- have low blood pressure
- have a slow heart rate
- have any heart problems, including history of heart attack or a condition called long QT syndrome
- have liver or kidney problems
- drink alcohol
- are pregnant or plan to become pregnant. It is not known if Lucemyra can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Lucemyra passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with Lucemyra.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements, and any medications you may take for the individual symptoms of opioid withdrawal (such as pain relievers or medications for upset stomach).
Especially tell your healthcare provider if you take benzodiazepines, barbiturates, tranquilizers, or sleeping pills. Taking Lucemyra with these medicines can cause serious side effects. Ask your healthcare provider or pharmacist if you are not sure if you are taking any of these medicines.
How should I take Lucemyra?
- Take Lucemyra exactly as your healthcare provider tells you to take it.
- Your healthcare provider may change your dose if needed.
- Do not change your dose or stop taking Lucemyra without talking to your healthcare provider.
- Take Lucemyra with or without food.
- If you take too much Lucemyra, go to the nearest hospital emergency room right away.
What should I avoid while taking Lucemyra?
Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how Lucemyra affects you.
What are the possible side effects of Lucemyra?
The most common side effects of Lucemyra include:
- low blood pressure or symptoms of low blood pressure such as lightheadedness
- slow heart rate
- dizziness
- sleepiness
- dry mouth
These are not all the possible side effects of Lucemyra.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to US WorldMeds at 1-833-LUCEMYRA.
General information about the safe and effective use of Lucemyra
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Lucemyra for a condition for which it was not prescribed. Do not give Lucemyra to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Lucemyra that is written for health professionals.
How should I store Lucemyra?
- Store Lucemyra at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Lucemyra in its original container.
- Keep Lucemyra away from heat and moisture.
- Lucemyra bottles contain desiccant packs to help keep the tablets dry. Do not remove the desiccant packs until all the tablets are used.
Keep Lucemyra and all medicines out of the reach of children.
What are the ingredients in Lucemyra?
Active ingredient: lofexidine.
Inactive ingredients: lactose, citric acid, povidone, microcrystalline cellulose, calcium stearate, sodium lauryl sulphate, and Opadry OY S 9480 (contains indigo carmine and sunset yellow).
Label
PRINCIPAL DISPLAY PANEL – 0.18 MG TABLET BOTTLE CARTON
- Rx only
NDC 78670-050-96 - Lucemyra®
(lofexidine) tablets - 0.18 mg
- Store and dispense
in original container. - Protect from heat and moisture.
Do not remove desiccants.
Keep the bottle tightly closed. - Keep out of reach of children.
96 tablets - US WorldMeds®


SRC: NLM .