Mayzent
Generic name: siponimod
Drug class: Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Mayzent?
Mayzent is a prescription medicine that is used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
It is not known if Mayzent is safe and effective in children.
Description
MAYZENT tablets contains siponimod, an S1P receptor modulator, as 2:1 co-crystal of siponimod and fumaric acid and has the following chemical name:
1-[[4-[(1E)-1-[[[4-Cyclohexyl-3-(trifluoromethyl)phenyl]methoxy]imino]ethyl]-2-ethylphenyl]methyl]-3-azetidinecarboxylic acid (2E)-2-butenedioate (2:1). Its molecular formula is C4H4O4•2C29H35F3N2O3, and its molecular weight is 1149.29 g/mol.
Its structure is shown below:
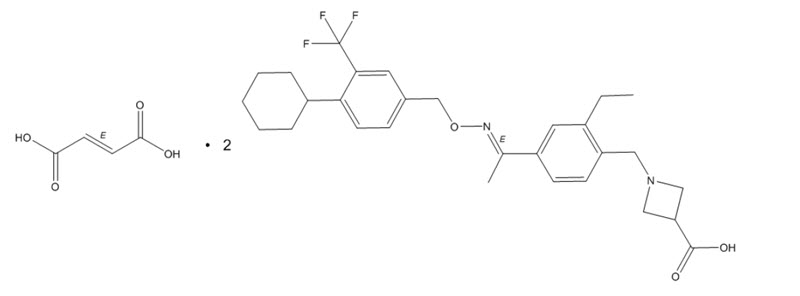
It is a white to almost white powder.
MAYZENT is provided as 0.25 mg, 1 mg, and 2 mg film-coated tablets for oral use. Each tablet contains 0.25 mg, 1 mg, or 2 mg siponimod, equivalent to 0.28 mg, 1.11 mg, or 2.22 mg as 2:1 co-crystal of siponimod and fumaric acid, respectively.
MAYZENT tablets contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, glyceryl dibehenate, lactose monohydrate, microcrystalline cellulose, with a film coating containing iron oxides (black and red iron oxides for the 0.25 mg and 1 mg strengths and red and yellow iron oxides for the 2 mg strength), lecithin (soy), polyvinyl alcohol, talc, titanium dioxide, and xanthan gum.
Mechanism of Action
Siponimod is an S1P receptor modulator. Siponimod binds with high affinity to S1P receptors 1 and 5. Siponimod blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which siponimod exerts therapeutic effects in multiple sclerosis is unknown, but may involve reduction of lymphocyte migration into the central nervous system.
What is the most important information I should know about Mayzent?
Mayzent may cause serious side effects, including:
1. Slow heart rate (bradycardia or bradyarrhythmia) when you start taking Mayzent. Mayzent can cause your heart rate to slow down, especially after you take your first dose. You should have a test to check the electrical activity of your heart called an electrocardiogram (ECG) before you take your first dose of Mayzent.
During the initial updosing period (4 days for the 1 mg daily dose or 5 days for the 2 mg daily dose), if you miss 1 or more doses of Mayzent, you need to restart the updosing. Call your healthcare provider if you miss a dose of Mayzent. See “How should I take Mayzent?”
2. Infections. Mayzent can increase your risk of serious infections that can be life-threatening and cause death. Mayzent lowers the number of white blood cells (lymphocytes) in your blood. This will usually go back to normal within 3 to 4 weeks of stopping treatment. Your healthcare provider should review a recent blood test of your white blood cells before you start taking Mayzent.
Call your healthcare provider right away if you have any of these symptoms of an infection during treatment with Mayzent and for 3 to 4 weeks after your last dose of Mayzent:
- fever
- tiredness
- body aches
- chills
- nausea
- vomiting
- headache with fever, neck stiffness, sensitivity to light, nausea, confusion (these may be symptoms of meningitis, an infection of the lining around your brain and spine)
3. A problem with your vision called macular edema. Macular edema can cause some of the same vision symptoms as a multiple sclerosis (MS) attack (optic neuritis). You may not notice any symptoms with macular edema. If macular edema happens, it usually starts in the first 1 to 4 months after your start taking Mayzent. Your healthcare provider should test your vision before you start taking Mayzent and any time you notice vision changes during treatment with Mayzent. Your risk of macular edema is higher if you have diabetes or have had an inflammation of your eye called uveitis.
Call your healthcare provider right away if you have any of the following:
- blurriness or shadows in the center of your vision
- a blind spot in the center of your vision
- sensitivity to light
- unusually colored (tinted) vision
See “What are possible side effects of Mayzent?” for more information about side effects.
Who should not take Mayzent?
Do not take Mayzent if you:
- have a CYP2C9*3/*3 genotype. Before starting treatment with Mayzent, your CYP2C9 genotype should be determined by your healthcare provider. Ask your healthcare provider if you are not sure.
- have had a heart attack, chest pain called unstable angina, stroke or mini-stroke (transient ischemic attack or TIA), or certain types of heart failure in the last 6 months
- have certain types of heart block or irregular or abnormal heartbeat (arrhythmia), unless you have a pacemaker
What should I tell my healthcare provider before taking Mayzent?
Before taking Mayzent, tell your healthcare provider about all of your medical conditions, including if you:
- have an irregular or abnormal heartbeat
- a history of stroke or other diseases related to blood vessels in the brain
- breathing problems, including during your sleep
- a fever or infection, or you are unable to fight infections due to a disease or taking medicines that lower your immune system. Tell your healthcare provider if you have had chicken pox or have received the vaccine for chicken pox. Your healthcare provider may do a blood test for chicken pox virus. You may need to get the full course of vaccine for chicken pox and then wait 1 month before you start taking Mayzent.
- have slow heart rate
- have liver problems
- have diabetes
- have eye problems, especially an inflammation of the eye called uveitis
- have high blood pressure
- are pregnant or plan to become pregnant. Mayzent may harm your unborn baby. Talk to your healthcare provider right away if you become pregnant while taking Mayzent or if you become pregnant within 10 days after you stop taking Mayzent.
- If you are a woman who can become pregnant, you should use effective birth control during your treatment with Mayzent and for at least 10 days after you stop taking Mayzent.
- are breastfeeding or plan to breastfeed. It is not known if Mayzent passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Mayzent.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you:
- take medicines to control your heart rhythm (antiarrhythmics), or blood pressure (antihypertensives), or heart beat (such as calcium channel blockers or beta-blockers)
- take medicines that affect your immune system, such as beta-interferon or glatiramer acetate, or any of these medicines that you took in the past
- have recently received a live vaccine. You should avoid receiving live vaccines during treatment with Mayzent. Mayzent should be stopped 1 week before and for 4 weeks after receiving a live vaccine. If you receive a live vaccine, you may get the infection the vaccine was meant to prevent. Vaccines may not work as well when given during treatment with Mayzent.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.
Using Mayzent and other medicines together may affect each other causing serious side effects.
How should I take Mayzent?
The daily maintenance dose of Mayzent is either 1 mg or 2 mg, depending on your CYP2C9 genotype. Ask your healthcare provider if you are not sure about your daily maintenance dose.
Do not split, crush, or chew Mayzent tablets; take tablets whole.
Start your treatment with Mayzent using the following titration schedule:
| For the 1 mg daily maintenance dose: | Tablets a day |
| Day 1 | 1 x 0.25 mg tablet |
| Day 2 | 1 x 0.25 mg tablet |
| Day 3 | 2 x 0.25 mg tablet |
| Day 4 | 3 x 0.25 mg tablet |
| Day 5 and everyday after | 4 x 0.25 mg tablet |
| For the 2 mg daily maintenance dose, use the starter pack: | Tablets a day |
| Day 1 | 1 x 0.25 mg tablet |
| Day 2 | 1 x 0.25 mg tablet |
| Day 3 | 2 x 0.25 mg tablet |
| Day 4 | 3 x 0.25 mg tablet |
| Day 5 | 5 x 0.25 mg tablet |
| Day 6 and everyday after | 1 x 2 mg tablet |
- Take Mayzent exactly as your healthcare provider tells you. Do not change your dose or stop taking Mayzent unless your healthcare provider tells you to.
- Take Mayzent 1 time each day.
- Take Mayzent with or without food.
- If you miss 1 or more doses of Mayzent during the initial dose titration, you need to restart the medication.
- If you miss a dose of Mayzent after the initial dose-titration, take it as soon as you remember.
- If Mayzent treatment is stopped for 4 days in a row, treatment has to be restarted with the titration.
- Do not stop taking Mayzent without talking with your healthcare provider first.
What are the possible side effects of Mayzent?
Mayzent may cause serious side effects, including:
- See “What is the most important information I should know about Mayzent?”
- increased blood pressure. Your healthcare provider should check your blood pressure during treatment with Mayzent.
- liver problems. Mayzent may cause liver problems. Your healthcare provider should do blood tests to check your liver before you start taking Mayzent. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
- nausea
- vomiting
- stomach pain
- tiredness
- loss of appetite
- your skin or the whites of your eyes turn yellow
- dark urine
- breathing problems. Some people who take Mayzent have shortness of breath. Call your healthcare provider right away if you have new or worsening breathing problems.
- swelling and narrowing of the blood vessels in your brain. A condition called PRES (Posterior Reversible Encephalopathy Syndrome) has happened with drugs in the same class. Symptoms of PRES usually get better when you stop taking Mayzent. However, if left untreated, it may lead to a stroke. Call your healthcare provider right away if you have any of the following symptoms:
- sudden severe headache
- sudden confusion
- sudden loss of vision or other changes in your vision
- seizure
- severe worsening of multiple sclerosis after stopping Mayzent. When Mayzent is stopped, symptoms of MS may return and become worse compared to before or during treatment. Always talk to your doctor before you stop taking Mayzent for any reason. Tell your healthcare provider if you have worsening symptoms of MS after stopping Mayzent.
The most common side effects of Mayzent include:
- headache
- high blood pressure (hypertension)
- abnormal liver tests
Tell your healthcare provider if you have any side effects that bother you or that do not go away.
These are not all of the possible side effects of Mayzent. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Mayzent
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Mayzent for a condition for which it was not prescribed. Do not give Mayzent to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for more information about Mayzent that is written for health professionals.
How should I store Mayzent?
Unopened Containers
Mayzent 0.25 mg and 2 mg tablets may be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 3 months. If you need to store Mayzent tablets for more than 3 months, containers should remain unopened and stored in a refrigerator between 36°F to 46°F (2°C to 8°C) until use.
Opened Containers
- Bottles
Mayzent 0.25 mg and 2 mg tablets may be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 3 months. Do not refrigerate after opening. - Starter Pack/Blister Card
Mayzent 0.25 mg tablets may be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 3 months. Do not refrigerate after opening. Store in original calendarized blister wallet container.
Keep Mayzent and all medicines out of the reach of children.
What are the ingredients in Mayzent?
Active ingredient: siponimod
Inactive ingredients: colloidal silicon dioxide, crospovidone, glyceryl behenate, lactose monohydrate, microcrystalline cellulose, with a film coating containing iron oxides (black and red iron oxides for the 0.25 mg strength and red and yellow iron oxides for the 2 mg strength), lecithin (soy), polyvinyl alcohol, talc, titanium dioxide, and xanthan gum.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-0979-50
- Rx only
- MAYZENT®
- (siponimod) tablets
- 0.25 mg*
- 28 tablets
- Dispense with accompanying Medication Guide.
- NOVARTIS

PRINCIPAL DISPLAY PANEL
- NDC 0078-1014-15
- Rx only
- MAYZENT®
- (siponimod) tablets
- 1 mg*
- 30 tablets
- Dispense with accompanying Medication Guide.
- Swallow tablets whole. DO NOT split tablets.
- NOVARTIS
PRINCIPAL DISPLAY PANEL
- NDC 0078-0986-15
- Rx only
- MAYZENT®
- (siponimod) tablets
- 2 mg*
- 30 tablets
- Dispense with accompanying Medication Guide.
- Swallow tablets whole. DO NOT split tablets.
- NOVARTIS

SRC: NLM .