Namenda
Generic name: memantine
Brand names: Namenda, Namenda XR
Drug class: Miscellaneous central nervous system agents
Medically reviewed by A Ras MD.
What is Namenda?
Namenda is a prescription medicine used for the treatment of moderate to severe dementia in people with Alzheimer’s disease. Namenda belongs to a class of medicines called NMDA (N-methyl-D-aspartate) inhibitors.
It is not known if Namenda is safe and effective in children.
Description
NAMENDA (memantine hydrochloride) is an orally active NMDA receptor antagonist. The chemical name for memantine hydrochloride is 1-amino-3,5-dimethyladamantane hydrochloride with the following structural formula:
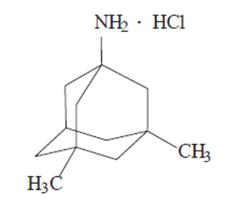
The molecular formula is C12H21N•HCl and the molecular weight is 215.76. Memantine hydrochloride occurs as a fine white to off-white powder and is soluble in water.
NAMENDA tablets are available for oral administration as capsule-shaped, film-coated tablets containing 5 mg and 10 mg of memantine hydrochloride. NAMENDA tablets also contain the following inactive ingredients: microcrystalline cellulose/colloidal silicon dioxide, talc, croscarmellose sodium, and magnesium stearate. In addition, the following inactive ingredients are also present as components of the film coat: hypromellose, titanium dioxide, polyethylene glycol 400, FD&C yellow #6 and FD&C blue #2 (5 mg tablets), and hypromellose, titanium dioxide, macrogol/polyethylene glycol 400 and iron oxide black (10 mg tablets).
Mechanism of Action
Persistent activation of central nervous system N-methyl-D-aspartate (NMDA) receptors by the excitatory amino acid glutamate has been hypothesized to contribute to the symptomatology of Alzheimer’s disease. Memantine is postulated to exert its therapeutic effect through its action as a low to moderate affinity uncompetitive (open-channel) NMDA receptor antagonist which binds preferentially to the NMDA receptor-operated cation channels. There is no evidence that memantine prevents or slows neurodegeneration in patients with Alzheimer’s disease.
Who should not take Namenda?
Do not take Namenda if you are allergic to memantine or any of the ingredients in Namenda. See the end of this guide for a complete list of ingredients in Namenda.
What should I tell my healthcare provider before taking Namenda?
Before you take Namenda, tell your doctor if you:
- have or have had seizures
- have or have had problems passing urine
- have or have had bladder or kidney problems
- have liver problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Namenda will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if memantine passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take Namenda.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking Namenda with certain other medicines may affect each other. Taking Namenda with other medicines can cause serious side effects.
Especially tell your doctor if you take:
- other NMDA antagonists such as amantadine, ketamine, and dextromethorphan
- medicines that make your urine alkaline such as carbonic anhydrase inhibitors and sodium bicarbonate
Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Namenda?
- Your doctor will tell you how much Namenda to take and when to take it.
- Your doctor may change your dose if needed.
- Namenda can be taken with food or without food.
- Do not use any tablets of Namenda that are damaged or show signs of tampering.
- If you forget to take one dose of Namenda, do not double up on the next dose. You should take only the next dose as scheduled.
- If you have forgotten to take Namenda for several days, you should not take the next dose until you talk to your doctor.
- If you take too much Namenda, call your doctor or poison control center at 1-800-222-1222 right away, or go to the nearest hospital emergency room.
What are the possible side effects of Namenda?
The most common side effects of Namenda include:
- dizziness
- nausea
- headache
- confusion
- constipation
These are not all the possible side effects of Namenda. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Namenda
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not take Namenda for a condition for which it was not prescribed. Do not give Namenda to other people, even if they have the same condition. It may harm them.
This Patient Information guide summarizes the most important information about Namenda. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Namenda that was written for healthcare professionals.
For more information about Namenda, go to www.Namenda.com, or call Allergan at 1-800-678-1605.
How should I store Namenda?
- Store Namenda at room temperature between 59°F to 77°F (15°C to 30°C).
Keep out of sight and reach of children.
What are the ingredients in Namenda?
Active ingredient: memantine hydrochloride
Inactive ingredients: microcrystalline cellulose/colloidal silicon dioxide, talc, croscarmellose sodium, and magnesium stearate
Inactive ingredients of tablet film coating: hypromellose, titanium dioxide, polyethylene glycol 400, FD&C yellow #6 and FD&C blue #2 (5 mg tablets), and hypromellose, titanium dioxide, macrogol/polyethylene glycol 400 and iron oxide black (10 mg tablets)
Label
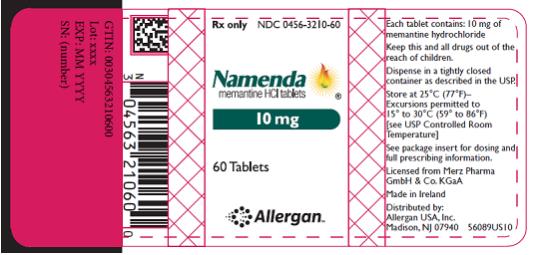
PRINCIPAL DISPLAY PANEL
- Rx Only NDC 0456-3205-60
- Namenda
- memantine HCl tablets
- 5 mg
- 60 Tablets
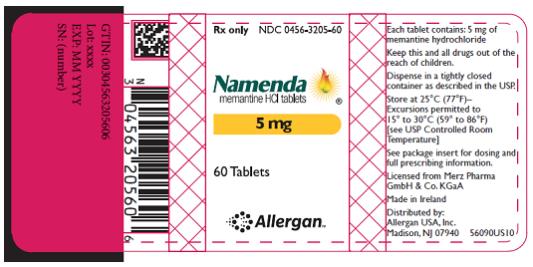

PRINCIPAL DISPLAY PANEL