Orilissa
Generic name: elagolix
Drug class: Gonadotropin-releasing hormone antagonists
Medically reviewed by A Ras MD.
What is Orilissa?
Orilissa is a prescription medicine used to treat moderate to severe pain associated with endometriosis. It is not known if Orilissa is safe and effective in children under 18 years of age.
Description
ORILISSA (elagolix) tablets for oral administration contain elagolix sodium, the sodium salt of the active moiety elagolix. Elagolix sodium is a nonpeptide small molecule, GnRH receptor antagonist. Elagolix sodium is chemically described as sodium 4-({(1R)-2-[5-(2-fluoro-3-methoxyphenyl)-3-{[2-fluoro-6-(trifluoromethyl)phenyl]methyl}-4-methyl-2,6-dioxo-3,6-dihydropyrimidin-1(2H)-yl]-1-phenylethyl}amino)butanoate. Elagolix sodium has a molecular formula of C32H29F5N3O5Na and a molecular weight of 653.58. Elagolix free acid has a molecular weight of 631.60.
Elagolix sodium has the following structural formula:
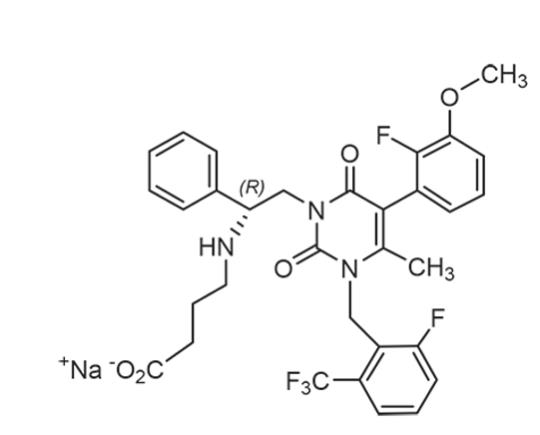
Elagolix sodium is a white to off white to light yellow powder and is freely soluble in water.
ORILISSA 150 mg tablets are light pink, oblong, film-coated tablets with “EL 150” debossed on one side. Each tablet contains 155.2 mg of elagolix sodium (equivalent to 150 mg of elagolix) as the active ingredient and the following inactive ingredients: mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and carmine high tint.
ORILISSA 200 mg tablets are light orange, oblong, film-coated tablets with “EL 200” debossed on one side. Each tablet contains 207.0 mg of elagolix sodium (equivalent to 200 mg of elagolix) as the active ingredient and the following inactive ingredients: mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
Mechanism of Action
ORILISSA is a GnRH receptor antagonist that inhibits endogenous GnRH signaling by binding competitively to GnRH receptors in the pituitary gland. Administration of ORILISSA results in dose-dependent suppression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to decreased blood concentrations of the ovarian sex hormones, estradiol and progesterone.
What is the most important information I should know about Orilissa?
Orilissa may cause serious side effects, including:
- bone loss (decreased bone mineral density).
- While you are taking Orilissa, your estrogen levels will be low. Low estrogen levels can lead to bone mineral density loss.
- Your bone density may improve after you stop taking Orilissa but complete recovery may not occur. It is unknown if these bone changes could increase your risk for broken bones as you age.
- Your healthcare provider may advise you to take vitamin D and calcium supplements as part of a healthy lifestyle that promotes bone health.
- If you have conditions or take other medicines that can cause bone loss, or if you have broken a bone with minimal or no injury, your healthcare provider may order an X-ray test called a DXA scan to check your bone mineral density.
- effects on pregnancy
- Do not take Orilissa if you are trying to become or are pregnant. It may increase the risk of early pregnancy loss.
- If you think you are pregnant, stop taking Orilissa right away and call your healthcare provider.
- If you become pregnant while taking Orilissa, you are encouraged to enroll in the Pregnancy Registry. The purpose of the pregnancy registry is to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-833-782-7241 to enroll in this registry.
- Orilissa may change your menstrual periods (irregular bleeding or spotting, a decrease in menstrual bleeding, or no bleeding at all), making it hard to know if you are pregnant. Watch for other signs of pregnancy such as breast tenderness, weight gain and nausea.
- Orilissa does not prevent pregnancy. You will need to use effective methods of birth control that do not contain hormones such as condoms or spermicide while taking Orilissa and for 1 week after you stop taking Orilissa. Birth control pills that contain estrogen may make Orilissa less effective. It is not known how well Orilissa will work while you are taking progestin-only birth control such as injections or implants.
- Talk to your healthcare provider about which birth control to use during treatment with Orilissa. Your healthcare provider may change the birth control you were on before you start taking Orilissa.
Who should not take Orilissa?
Do not take Orilissa if you:
- are or may be pregnant
- have osteoporosis
- have severe liver disease
- are taking medicines known as strong OATP1B1 inhibitors such as cyclosporine or gemfibrozil. Ask your healthcare provider if you are not sure if you are taking one of these medicines.
What should I tell my healthcare provider before taking Orilissa?
Before you take Orilissa, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had broken bones
- have other conditions or take medicines that may cause bone problems
- have or have had depression, mood problems or suicidal thoughts or behavior
- have liver problems
- think you may be pregnant. You should avoid becoming pregnant while taking Orilissa
- are breastfeeding or plan to breastfeed. It is not known if Orilissa passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby if you take Orilissa.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- birth control pills. Your healthcare provider may advise you to change the pills you take, or your method of birth control.
Know the medicines you take. Keep a list of your medicines with you to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Orilissa?
- Take Orilissa exactly as your healthcare provider tells you to take it.
- Your healthcare provider will give you a pregnancy test before you start taking Orilissa or will have you start taking Orilissa within 7 days after you start your period.
- If your healthcare provider prescribes: Orilissa 150 mg (a pink tablet), take it 1 time each day
- Orilissa 200 mg (an orange tablet), take it 2 times each day
- Take Orilissa at about the same time each day with or without food.
- If you miss a dose of Orilissa:
- 150 mg (1 time each day), take it as soon as you remember as long as it is on the same day. Do not take more than 1 tablet each day.
- 200 mg (2 times each day), take it as soon as you remember as long as it is on the same day. Do not take more than 2 tablets each day.
- If you take too much Orilissa, call your healthcare provider or go to the nearest hospital.
What are the possible side effects of Orilissa?
Orilissa can cause serious side effects including:
- See “What is the most important information I should know about Orilissa?”
- suicidal thoughts, suicidal behavior, and worsening of mood. Orilissa may cause suicidal thoughts or actions. Call your healthcare provider right away if you have any of these symptoms or call 911 if an emergency, especially if they are new, worse, or bother you:
- thoughts about suicide or dying
- try to commit suicide
- new or worse depression
- new or worse anxiety
- other unusual changes in behavior or mood
You or your caregiver should pay attention to any changes, especially sudden changes in your mood, behaviors, thoughts, or feelings.
- abnormal liver tests. Call your healthcare provider right away if you have any of these signs and symptoms of liver problems:
- yellowing of the skin or the whites of the eyes (jaundice)
- dark amber-colored urine
- feeling tired (fatigue or exhaustion)
- nausea and vomiting
- generalized swelling
- right upper stomach area (abdomen) pain
- bruising easily
The most common side effects of Orilissa include: hot flashes or night sweats, headache, nausea, difficulty sleeping, absence of periods, anxiety, joint pain, depression and mood changes.
These are not all the possible side effects of Orilissa. Call your healthcare provider for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Orilissa
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Orilissa for a condition for which it was not prescribed. Do not give Orilissa to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Orilissa that is written for health professionals.
How should I store Orilissa?
- Store Orilissa between 36°F to 86°F (2°C to 30°C).
- Do not keep medicine that is out of date or that you no longer need. Dispose of unused medicines through community take-back disposal programs when available or place Orilissa in an unrecognizable closed container in the household trash. Do NOT flush Orilissa down the toilet. See www.fda.gov/drugdisposal for more information.
- Keep Orilissa and all medicines out of the reach of children.
What are the ingredients in Orilissa?
Active ingredient: elagolix
Inactive ingreadients
150 mg tablets: mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and carmine high tint.
200 mg tablets: mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
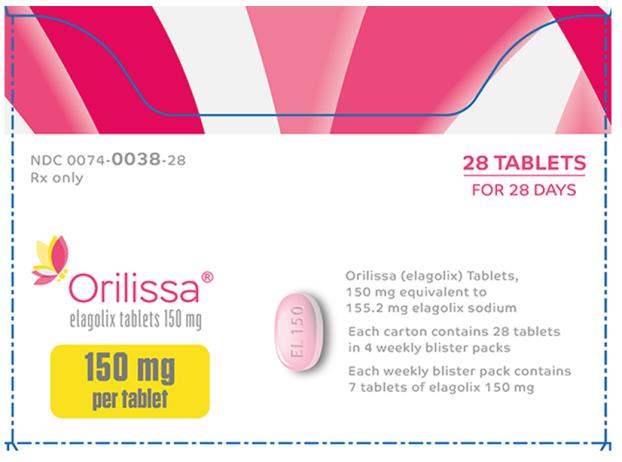
Label
PRINCIPAL DISPLAY PANE
- NDC 0074-0038-28
- Rx only
- 28 TABLETS
- FOR 28 DAYS
- Orilissa®
- elagolix tablets 150 mg
- 150 mg per tablet
- Orilissa (elagolix) Tablets,
- 150 mg equivalent to 155.2 mg elagolix sodium
- Each carton contains 28 tablets in 4 weekly blister packs
- Each weekly blister pack contains 7 tablets of elagolix 150 mg

PRINCIPAL DISPLAY PANEL
- NDC 0074-0039–01
- Rx only
- 14 TABLETS
- FOR 7 DAYS
- Orilissa®
- elagolix tablets 200 mg
- 200 mg per tablet
- Orilissa (elagolix) Tablets,
- 200 mg equivalent to 207.0 mg elagolix sodium

SRC: NLM .
