Osmolex ER
Generic name: amantadine
Brand names: Gocovri, Osmolex ER
Drug class: Dopaminergic antiparkinsonism agents
Medically reviewed by A Ras MD.
What is Osmolex ER?
Osmolex ER is a prescription medicine used to treat Parkinson’s disease. it is also used for the treatment of drug-induced extrapyramidal reactions in adult patients
It is not known if Osmolex ER is safe and effective in children.
Description
OSMOLEX ER contains amantadine in an extended-release tablet. The active ingredient in OSMOLEX ER is amantadine hydrochloride.
The chemical name for amantadine hydrochloride is tricyclo [3.3.1.1 3,7] decan-1-amine, hydrochloride or 1-adamantanamine hydrochloride, and it has the following structural formula:
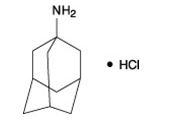
The molecular formula is C10H17N•HCl and the molecular weight is 187.71 (g/mol). Amantadine hydrochloride, USP is a stable white or nearly white crystalline powder, freely soluble in water and alcohol, and soluble in chloroform.
OSMOLEX ER tablets are for oral use. Each tablet contains 129 mg, or 193 mg amantadine (as 160 mg or 240 mg, amantadine hydrochloride, respectively) in an extended-release core and an outer immediate-release layer. Amantadine release from the extended-release core is controlled by an osmotic pump system. Osmotic pump systems consist of a drug core contained within a semipermeable polymer membrane that is permeable to water molecules, but not to the drug, with a laser drilled orifice for drug delivery. Amantadine release is driven by the existence of an osmotic gradient between the contents of the drug core and the fluid in the gastrointestinal tract. Since the osmotic gradient remains constant, drug delivery remains essentially constant after the immediate-release layer dissolves. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the stool as a tablet shell.
Inactive ingredients: cellulose acetate, colloidal silicon dioxide, copovidone, D&C Yellow No. 10, FD&C Yellow No. 6, ferrosoferric oxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, propylene glycol, sodium chloride, and titanium dioxide. OSMOLEX ER 129 mg tablets also contain lactose monohydrate and triacetin. OSMOLEX ER 193 mg tablets also contain FD&C Blue No. 2 and FD&C Red No. 40.
Mechanism of Action
The mechanism by which amantadine exerts efficacy in the treatment of Parkinson’s disease and drug-induced extrapyramidal reactions is unknown. Amantadine is a weak uncompetitive antagonist of the NMDA receptor; however, it exhibits anticholinergic-like side effects such as dry mouth, urinary retention, and constipation in humans. Amantadine may have direct and indirect effects on dopamine neurons; it exerts dopaminergic-like side effects such as hallucinations and dizziness in humans.
Who should not take Osmolex ER?
Do not take Osmolex ER if you have severe kidney problems.
What should I tell my healthcare provider before taking Osmolex ER?
Before taking Osmolex ER, tell your doctor about all of your medical conditions, including if you:
- have kidney problems.
- have daytime sleepiness from a sleep disorder, have unexpected or unpredictable sleepiness or periods of sleep, take a medicine to help you sleep, or take any medicine that makes you drowsy.
- have mental health problems, such as suicidal thoughts, depression, or hallucinations.
- have unusual urges including gambling, increased sex drive, compulsive eating, or compulsive shopping.
- drink alcohol. Drinking alcohol may increase your chances of becoming drowsy, sleepy, confused, dizzy, light headed, or faint while taking Osmolex ER.
- are pregnant or plan to become pregnant. Osmolex ER may harm your unborn baby.
- are breastfeeding or plan to breastfeed. Osmolex ER can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you take Osmolex ER.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and dietary and herbal supplements. Osmolex ER may affect the way certain other medicines work, and certain other medicines can affect how Osmolex ER works.
Especially tell your doctor if you:
- take sodium bicarbonate or a medicine that contains sodium bicarbonate.
- are planning to receive a flu (influenza) vaccine. You may receive the flu vaccine shot but should not get a live flu vaccine (nasal spray) while taking Osmolex ER.
How should I take Osmolex ER?
- Take Osmolex ER exactly as your doctor tells you to.
- Start Osmolex ER with 1 tablet in the morning. Your doctor may change your dose if needed.
- Do not stop taking Osmolex ER or change your dose before talking with your doctor. Tell your doctor if you have symptoms of withdrawal such as fever, confusion, or severe muscle stiffness.
- Osmolex ER may be taken with food or without food.
- Swallow Osmolex ER tablets whole. Do not chew, crush, or divide Osmolex ER tablets.
- If you miss a dose of Osmolex ER, do not make up the missed dose. Take your usual dose of Osmolex ER on the next day in the morning.
- If you take too much Osmolex ER, call your doctor or go to the nearest hospital emergency room right away.
- Osmolex ER should not be used instead of other immediate-release amantadine medicines or other extended-release amantadine medicines.
- The Osmolex ER tablet shell does not dissolve completely even after all the drug has been released. The tablet shell may be seen in your stool.
What should I avoid while taking Osmolex ER?
- Do not drive, operate machinery, or do other dangerous activities until you know how Osmolex ER affects you. If you have become sleepy during the day or have fallen asleep while doing normal daily activities during treatment with Osmolex ER, do not drive, operate machinery, or do other dangerous activities that could cause harm if you become sleepy.
- You should not drink alcohol during treatment with Osmolex ER. Drinking alcohol can increase your chances of getting serious side effects.
What are the possible side effects of Osmolex ER?
Osmolex ER may cause serious side effects, including:
- falling asleep during normal activities. You may fall asleep while doing normal activities such as driving a car, talking, or eating while taking Osmolex ER. You may fall asleep without being drowsy or without warning. This may result in having accidents. Your chances of falling asleep while doing normal activities while taking Osmolex ER are greater if you take other medicines that cause drowsiness or drink alcohol. Tell your doctor right away if this happens.
- suicidal thoughts or actions and depression. Some people taking amantadine who have a history of mental illness and some people who do not have a history of mental illness, have had depression, suicidal thoughts, attempted suicide, and have committed suicide. Amantadine can make psychiatric symptoms in people with a history of mental health problems or a history of substance abuse worse. Tell your doctor right away if you have changes in your mood, behaviors, or thoughts, including thoughts about hurting yourself or ending your life.
- seeing, hearing, or feeling things that are not real (hallucinations) and behaviors of being out of touch with reality (psychotic behaviors). Taking amantadine or stopping amantadine suddenly can cause confusion, psychosis, changes in personality, agitation, aggressiveness, hallucinations, and not trusting and being suspicious of others for no reason (paranoia). Tell your doctor right away if you have any of these changes in your behavior.
- feeling dizzy, faint or light headed, especially when you stand up (orthostatic hypotension). Dizziness, light headedness, or fainting can happen with Osmolex ER when getting up too quickly from a sitting or lying position, especially after a long period of time, and especially when first starting Osmolex ER or if your dose has been increased. Stand up slowly when moving from a sitting or lying position. Tell your doctor if you become dizzy, light headed or faint when standing up.
- unusual urges. Some people taking Osmolex ER get urges to behave in a way unusual for them. Examples of this are strong urges to gamble, increased sexual urges, strong urges to spend money, binge eating and the inability to control these urges. If you notice or your family notices that you are developing any unusual behaviors, talk to your doctor.
The most common side effects of Osmolex ER include nausea, dizziness, lightheadedness, and trouble sleeping (insomnia).
These are not all the possible side effects of Osmolex ER. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Osmolex ER
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Osmolex ER for a condition for which it was not prescribed. Do not give Osmolex ER to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about Osmolex ER that is written for health professionals.
How should I store Osmolex ER?
- Store Osmolex ER at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Osmolex ER and all medicines out of the reach of children.
What are the ingredients in Osmolex ER?
Active ingredient: amantadine hydrochloride
Inactive ingredients: cellulose acetate, colloidal silicon dioxide, copovidone, D&C Yellow No. 10, FD&C Yellow No. 6, ferrosoferric oxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, propylene glycol, sodium chloride, and titanium dioxide.
- Osmolex ER 129 mg tablets also contain lactose monohydrate and triacetin.
- Osmolex ER 193 mg tablets also contain FD&C Blue No. 2 and FD&C Red No. 40.
- Osmolex ER 258 mg tablets also contain FD&C Blue No. 1, FD&C Red No. 40, and ferric oxide yellow.
Label
PRINCIPAL DISPLAY PANEL
- NDC 70482-075-30
- Osmolex® ER
- 129 mg 30 Tablets Bottle Label

PRINCIPAL DISPLAY PANEL
- NDC 70482-075-07
- Osmolex® ER
- 129 mg Sample Package, 7 Tablets per Blister, 2 Blisters in a Carton

PRINCIPAL DISPLAY PANEL
