Padcev
Generic name: enfortumab vedotin
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Padcev?
Padcev is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter or urethra) that has spread or cannot be removed by surgery. Padcev may be used if you have received an immunotherapy medicine and lso received a chemotherapy-containing platinum medicine.
It is not known if Padcev is safe and effective in children.
Description
Enfortumab vedotin-ejfv is a Nectin-4 directed antibody-drug conjugate (ADC) comprised of a fully human anti-Nectin-4 IgG1 kappa monoclonal antibody (AGS-22C3) conjugated to the small molecule microtubule disrupting agent, monomethyl auristatin E (MMAE) via a protease-cleavable maleimidocaproyl valine-citrulline (vc) linker (SGD-1006). Conjugation takes place on cysteine residues that comprise the interchain disulfide bonds of the antibody to yield a product with a drug-to-antibody ratio of approximately 3.8:1. The molecular weight is approximately 152 kDa.
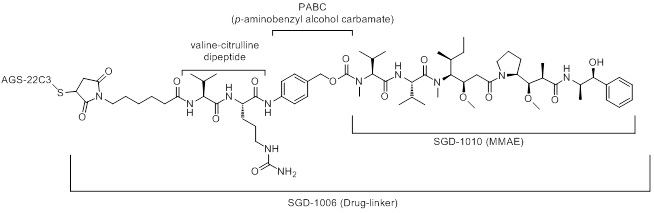
Figure 1. Structural Formula
Approximately 4 molecules of MMAE are attached to each antibody molecule. Enfortumab vedotin-ejfv is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells and the small molecule components are produced by chemical synthesis.
PADCEV (enfortumab vedotin-ejfv) for injection is provided as a sterile, preservative-free, white to off-white lyophilized powder in single-dose vials for intravenous use. PADCEV is supplied as a 20 mg per vial and a 30 mg per vial and requires reconstitution with Sterile Water for Injection, USP, (2.3 mL and 3.3 mL, respectively) resulting in a clear to slightly opalescent, colorless to slightly yellow solution with a final concentration of 10 mg/mL [see Dosage and Administration (2.3)]. After reconstitution, each vial allows the withdrawal of 2 mL (20 mg) and 3 mL (30 mg). Each mL of reconstituted solution contains 10 mg of enfortumab vedotin-ejfv, histidine (1.4 mg), histidine hydrochloride monohydrate (2.31 mg), polysorbate 20 (0.2 mg) and trehalose dihydrate (55 mg) with a pH of 6.0.
Mechanism of Action
Enfortumab vedotin-ejfv is an ADC. The antibody is a human IgG1 directed against Nectin-4, an adhesion protein located on the surface of cells. The small molecule, MMAE, is a microtubule-disrupting agent, attached to the antibody via a protease-cleavable linker. Nonclinical data suggest that the anticancer activity of enfortumab vedotin-ejfv is due to the binding of the ADC to Nectin-4-expressing cells, followed by internalization of the ADC-Nectin-4 complex, and the release of MMAE via proteolytic cleavage. Release of MMAE disrupts the microtubule network within the cell, subsequently inducing cell cycle arrest and apoptotic cell death.
What should I tell my healthcare provider before using Padcev?
Before receiving Padcev, tell your healthcare provider about all of your medical conditions, including if you:
- are currently experiencing numbness or tingling in your hands or feet
- have a history of high blood sugar or diabetes
- are pregnant or plan to become pregnant. Padcev can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Padcev.
- Females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with Padcev.
- You should use an effective method of birth control during your treatment and for at least 2 months after the last dose of Padcev.
- Males with a female sexual partner who is able to become pregnant:
- If your female partner is pregnant, Padcev can harm the unborn baby.
- You should use an effective method of birth control during your treatment and for at least 4 months after the last dose of Padcev.
- are breastfeeding or plan to breastfeed. It is not known if Padcev passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of Padcev.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Padcev?
- Padcev will be given to you by intravenous (IV) infusion into your vein over 30 minutes.
- You will receive your Padcev over periods of time called cycles.
- Each Padcev cycle is 28 days.
- You will receive Padcev on days 1, 8 and 15 of every cycle.
- Your healthcare provider will decide how many treatment cycles you need.
- Your healthcare provider may do blood tests regularly during treatment with Padcev.
What are the possible side effects of Padcev?
Padcev may cause serious side effects, including:
- High blood sugar (hyperglycemia). You can develop high blood sugar during treatment with Padcev. Tell your healthcare provider right away if you have any symptoms of high blood sugar, including:
- frequent urination
- increased thirst
- blurred vision
- confusion
- it becomes harder to control your blood sugar
- drowsiness
- loss of appetite
- fruity smell on your breath
- nausea, vomiting, or stomach pain
- Peripheral neuropathy. While receiving Padcev you may experience nerve problems called peripheral neuropathy. Tell your healthcare provider right away if you get numbness or tingling in your hands or feet or muscle weakness.
- Eye problems. You can develop certain eye problems while receiving Padcev. Tell your healthcare provider right away if you have dry eyes, or blurred vision.
- Skin Reactions. Rashes and severe skin reactions can happen while receiving Padcev. Tell your healthcare provider right away if you get a rash or a skin reaction that continues to get worse.
- Leakage of Padcev out of your vein into the tissues around your infusion site (extravasation). If Padcev leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, or discomfort at the infusion site.
The most common side effects of Padcev include:
- numbness or tingling in your hands or feet, or muscle weakness
- fatigue
- decreased appetite
- rash
- hair loss
- nausea
- diarrhea
- change in sense of taste
- dry eyes
- dry skin
If you have certain side effects, your healthcare provider may decrease your dose or stop your treatment with Padcev for a period of time (temporarily) or completely.
Padcev may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of Padcev.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Padcev
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. If you would like more information about Padcev, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Padcev that is written for healthcare professionals.
What are the ingredients in Padcev?
Active ingredient: enfortumab vedotin
Inactive ingredients: histidine, histidine hydrochloride monohydrate, polysorbate 20, and trehalose dehydrate.
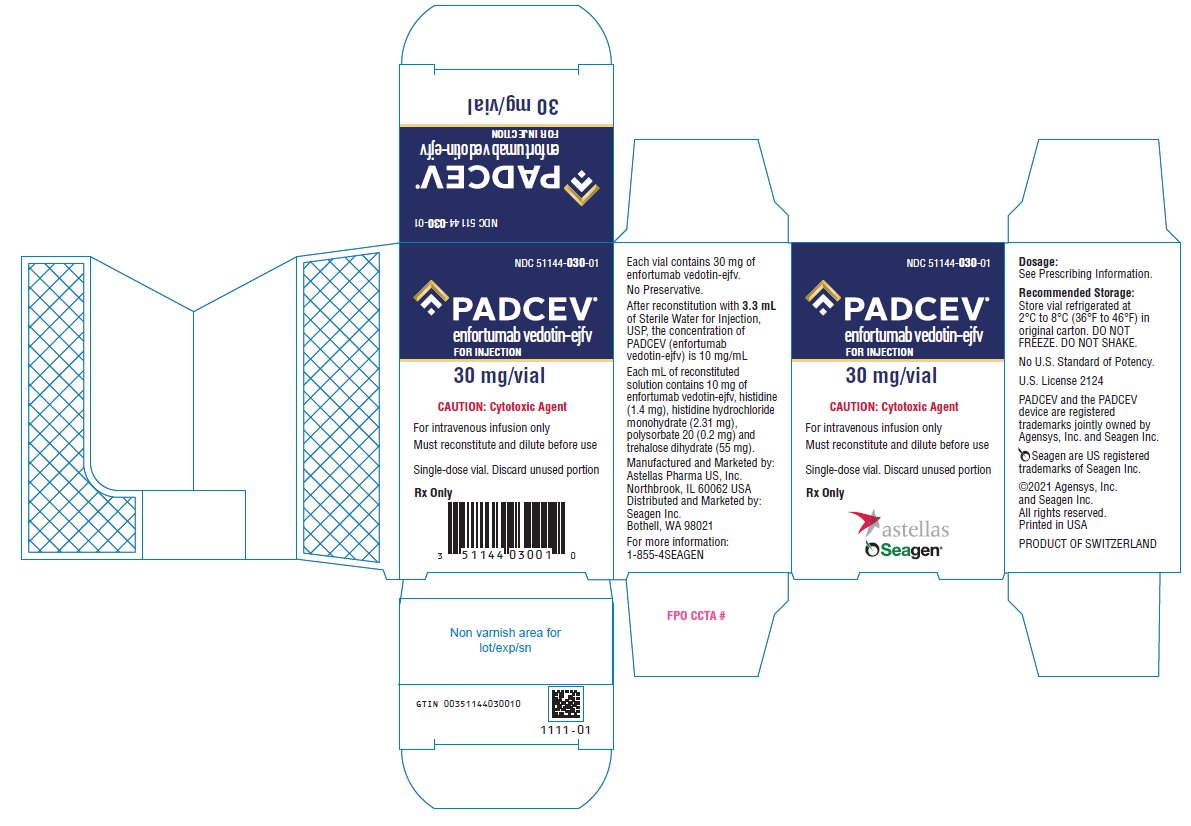
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 51144-020-01
- PADCEV®
- enfortumab vedotin-ejfv
- FOR INJECTION
- 20 mg/vial
- CAUTION: Cytotoxic Agent
- For intravenous infusion use only
- Must reconstitute and dilute before use
- Single-dose vial. Discard unused portion
- Rx Only
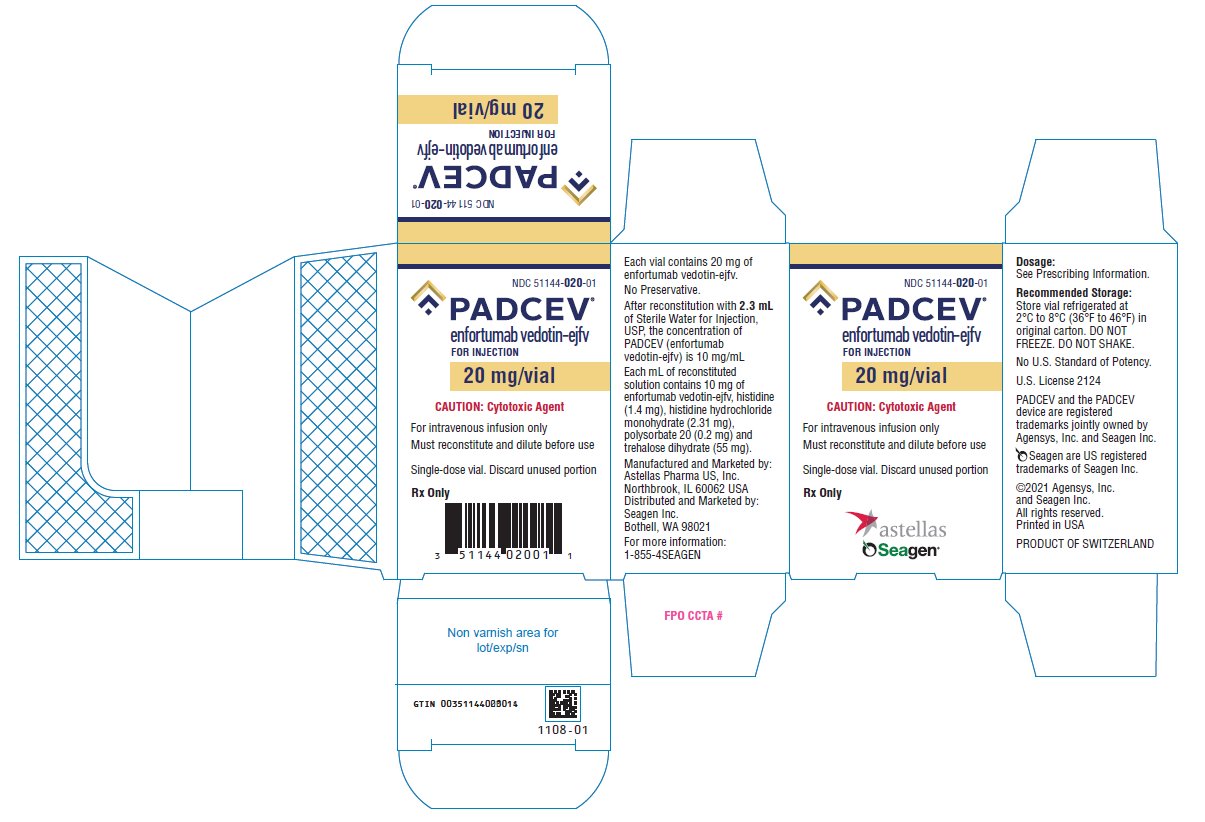
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 51144-030-01
- PADCEV®
- enfortumab vedotin-ejfv
- FOR INJECTION
- 30 mg/vial
- CAUTION: Cytotoxic Agent
- For intravenous infusion use only
- Must reconstitute and dilute before use
- Single-dose vial. Discard unused portion
- Rx Only