Pulmicort Flexhaler
Generic name: budesonide inhalation
Brand names: Pulmicort Flexhaler, Pulmicort Respules
Drug class: Inhaled corticosteroids
Medically reviewed by A Ras MD.
What is Pulmicort Flexhaler?
Pulmicort Flexhaler is an inhaled corticosteroid medicine. Pulmicort Flexhaler is used for long-term (maintenance) treatment of asthma and to prevent asthma symptoms in adults and children 6 years of age and older.
Inhaled corticosteroids help to decrease inflammation in the lungs. Inflammation in the lungs can lead to asthma symptoms.
Pulmicort Flexhaler helps reduce inflammation and helps keep the airways open to reduce asthma symptoms.
Pulmicort Flexhaler does not treat the symptoms of a sudden asthma attack. Always have a short-acting beta2-agonist medicine (rescue inhaler) with you to treat sudden symptoms. If you do not have an inhaled, short-acting bronchodilator, call your healthcare provider to have one prescribed for you.
It is not known if Pulmicort Flexhaler is safe and effective in children younger than 6 years of age.
Description
Budesonide, the active component of PULMICORT FLEXHALER, is a corticosteroid designated chemically as (RS)-11β, 16α, 17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
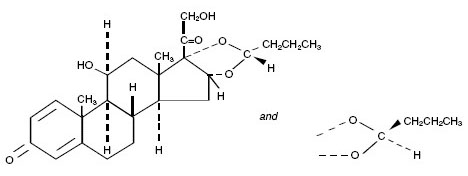
Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water and in heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 7.4 is 1.6 x 103.
PULMICORT FLEXHALER is an inhalation-driven multi-dose dry powder inhaler containing a formulation of 1 mg per actuation of micronized budesonide and micronized lactose monohydrate which contains trace levels of milk proteins. Each actuation of PULMICORT FLEXHALER 180 mcg delivers 160 mcg budesonide from the mouthpiece and each actuation of PULMICORT FLEXHALER 90 mcg delivers 80 mcg budesonide from the mouthpiece (based on in vitro testing at 60 L/min for 2 sec). Each PULMICORT FLEXHALER 180 mcg contains 120 actuations and each PULMICORT FLEXHALER 90 mcg contains 60 actuations.
In vitro testing has shown that the dose delivery for PULMICORT FLEXHALER is dependent on airflow through the device, as evidenced by a decrease in the fine particle dose at a flow rate of 30 L/min to a value that is approximately 40-50% of that produced at 60 L/min. At a flow rate of 40 L/min, the fine particle dose is approximately 70% of that produced at 60 L/min. Patient factors such as inspiratory flow rates will also affect the dose delivered to the lungs of patients in actual use. In asthmatic children age 6 to 17 (N=516, FEV1 2.29 [0.97– 4.28]) peak inspiratory flow (PIF) through PULMICORT FLEXHALER was 72.5 [19.1 – 103.6] L/min). Inspiratory flows were not measured in the adult pivotal study. Patients should be carefully instructed on the use of this drug product to assure optimal dose delivery.
Mechanism of Action
Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol (rat croton oil ear edema assay). As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay. The clinical significance of this is unknown.
The activity of PULMICORT FLEXHALER is due to the parent drug, budesonide. In glucocorticoid receptor affinity studies, the 22R form was two times as active as the 22S epimer. In vitro studies indicated that the two forms of budesonide do not interconvert.
The precise mechanism of corticosteroid actions on inflammation in asthma is not known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have a wide range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic and non-allergic-mediated inflammation. These anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
Studies in asthmatic patients have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects over a wide range of doses of inhaled budesonide. This is explained by a combination of a relatively high local anti-inflammatory effect, extensive first pass hepatic degradation of orally absorbed drug (85-95%), and the low potency of formed metabolites (see below).
What is the most important information I should know about Pulmicort Flexhaler?
This medicine is to only be inhaled through the mouth (by oral inhalation only).
Who should not use Pulmicort Flexhaler?
Do not use Pulmicort Flexhaler:
- to treat sudden severe symptoms of asthma.
- if you have a severe allergy to milk proteins. Pulmicort Flexhaler contains a small amount of lactose (milk sugar). People with severe allergies to milk protein may have symptoms of an allergic reaction with Pulmicort Flexhaler including: cough, wheezing, trouble breathing or feeling like your throat is closing.
What should I tell my healthcare provider before using Pulmicort Flexhaler?
Before using Pulmicort Flexhaler, tell your healthcare provider if you:
- have any allergies. See the section “Who should not use Pulmicort Flexhaler”. There is a complete list of ingredients in Pulmicort Flexhaler at the end of this leaflet.
- have or had chicken pox or measles, or have recently been near anyone with chicken pox or measles.
- have or had tuberculosis of your respiratory tract.
- have certain kinds of serious infections that have not been treated, including:
- fungal infections
- bacterial infections
- viral infections
- parasitic infections
- herpes simplex infection of the eye (ocular herpes simplex)
Pulmicort Flexhaler may not be right for people who have or had any of these types of infections.
- have liver problems
- have decreased bone mineral density.
You are at risk for decreased bone mineral density if you:
- are inactive for a long period of time
- have a family history of osteoporosis
- are a woman going through menopause or are past menopause (“the change of life”)
- smoke or use tobacco
- do not eat well (poor nutrition)
- are elderly
- take bone thinning medicines (such as anticonvulsant medicines or corticosteroids) for a long time.
- have eye problems such as increased pressure in the eye, glaucoma, or cataracts
- are planning to have surgery
- are pregnant or plan to become pregnant. It is not known if Pulmicort Flexhaler may harm your unborn baby
- are breast-feeding or plan to breast-feed. Pulmicort Flexhaler can pass into breast milk. You and your healthcare provider should decide if you will use Pulmicort Flexhaler or breast-feed
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Using Pulmicort Flexhaler with certain other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- a corticosteroid medicine
- anti-seizure medicine (anticonvulsants)
- medicines that suppress your immune system (immunosuppressant)
- ketoconazole (Nizoral), other medicines that affect how your liver works.
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one listed above.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use Pulmicort Flexhaler?
Use Pulmicort Flexhaler exactly as prescribed by your healthcare provider. You must use Pulmicort Flexhaler regularly for it to work.
- Pulmicort Flexhaler comes in two strengths. Your healthcare provider has prescribed the strength that is best for you.
- Be sure you know the difference between Pulmicort Flexhaler and any other inhaled medicines that are prescribed for you, including what you use them for (prescribed use) and what they look like.
- Do not stop using Pulmicort Flexhaler, even if your symptoms get better. Your healthcare provider will change your medicines as needed.
- Do not change or stop any medicines used to control or treat your breathing problems, unless your healthcare provider tells you to.
- Rinse your mouth with water and spit the water out after each dose of Pulmicort Flexhaler. Do not swallow the water. This will lessen the chance of getting a fungal infection (thrush) in the mouth.
- If you miss a dose, just take your next regularly scheduled dose when it is due. Do not use Pulmicort Flexhaler more often or use more puffs than you have been prescribed.
- Make sure you always have a short-acting beta2-agonist medicine with you. Use your short acting beta2-agonist medicine if you have breathing problems between doses of Pulmicort Flexhaler or if a sudden asthma attack happens. Call your healthcare provider right away if:
- your short-acting rescue medicine does not work as well for relieving asthma symptoms.
- you need to use your short-acting rescue medicines more often than usual.
- your breathing problems worsen with Pulmicort Flexhaler.
If you use another inhaled medicine by mouth to treat your asthma, talk with your healthcare provider for instructions about when to use the other medicine and when to use your Pulmicort Flexhaler.
- If you have used corticosteroid medicines for a long time and the dose is now being lowered or stopped, you should carry a medical alert card. The medical alert card should state that you may need increased corticosteroids during times of stress or during an asthma attack that does not get better with bronchodilator medicines.
- Your healthcare provider may check your breathing, do blood tests and eye exams during treatment with Pulmicort Flexhaler.
- Be sure to read, understand and follow the detailed Patient Instructions for Use at the end of this leaflet. These Instructions for Use tell you how to prime and use your Pulmicort Flexhaler the right way.
What are the possible side effects of Pulmicort Flexhaler?
Pulmicort Flexhaler can cause serious side effects, including:
- thrush (candida), a fungal infection in your mouth and throat. Tell your healthcare provider if you have any redness or white colored patches in your mouth or throat.
- worsening of asthma or sudden asthma attacks.
- allergic reactions. Tell your healthcare provider or get medical help right away if you have:
- skin rash, redness or swelling
- severe itching
- swelling of the face, mouth, and tongue
- trouble breathing or swallowing
- chest pain
- anxiety (feeling of doom)
- Immune system effects and a higher chance of infections. You are more likely to get infections if you take medicines that weaken your immune system. Avoid contact with people who have contagious diseases such as chicken pox or measles while using Pulmicort Flexhaler. Symptoms of infection may include: fever, pain, aches, chills, feeling tired, nausea and vomiting. Tell your healthcare provider about any signs of infection while you are using Pulmicort Flexhaler.
- Adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal insufficiency include: tiredness, weakness, nausea and vomiting and low blood pressure.
- Decrease in bone mineral density. Your healthcare provider should check you for this during treatment with Pulmicort Flexhaler.
- Slowed or delayed growth problems in children. A child’s growth should be checked regularly while using Pulmicort Flexhaler.
- Eye problems, including glaucoma and cataracts. You should have regular eye exams while using Pulmicort Flexhaler.
- Increased wheezing right after taking Pulmicort Flexhaler. Always have a short-acting beta2-agonist medicine (rescue inhaler) with you to treat sudden wheezing.
Call your healthcare provider or get medical help right away if you have symptoms of any of the serious side effects listed above.
Common side effects reported by patients using Pulmicort Flexhaler include:
- sore nose and throat
- stuffy nose
- runny nose
- nausea
- hay fever
- viral infections of the upper respiratory tract
- viral irritation and inflammation of the stomach and intestine (gastroenteritis). Symptoms may include stomach area pain, diarrhea, nausea and vomiting, loss of appetite, headaches, and weakness.
- ear infections
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all of the side effects of Pulmicort Flexhaler. Ask your healthcare provider or pharmacist for more information.
Call your healthcare provider for medical advice about side effects. You may report side effects to AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
General information about the safe and effective use of Pulmicort Flexhaler
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information Leaflets. Do not use Pulmicort Flexhaler for a condition for which it was not prescribed. Do not give Pulmicort Flexhaler to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Pulmicort Flexhaler. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Pulmicort Flexhaler that is written for health professionals.
For more information, go to PULMICORTFLEXHALER.COM or call 1- 800-236-9933.
How should I store Pulmicort Flexhaler?
Store Pulmicort Flexhaler at 68° to 77°F (20° to 25°C).
- Keep Pulmicort Flexhaler dry.
- Keep your Pulmicort Flexhaler with the cover tightly in place when not in use.
Keep your Pulmicort Flexhaler and all medicines out of the reach of children.
What are the ingredients in Pulmicort Flexhaler?
Active ingredient: budesonide
Inactive ingredient: lactose
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 90 MCG
- NDC 0186-0917-06
- Pulmicort
- Flexhaler™ 90 mcg
- (budesonide inhalation
- powder, 90 mcg)
- 90 mcg
- For Oral Inhalation.
- 60 Metered Doses
- Dispense with enclosed Patient
- Information and Instructions for Use.
- Rx only
- AstraZeneca
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 180 MCG
- NDC 0186-0916-12
- Pulmicort
- Flexhaler™ 180 mcg
- (budesonide inhalation
- powder, 180 mcg)
- 180 mcg
- For Oral Inhalation.
- 120 Metered Doses
- Dispense with enclosed Patient
- Information and Instructions for Use.
- Rx only
- AstraZeneca

SRC: NLM .