Qelbree
Generic name: viloxazine
Dosage form: extended-release capsules
Drug class: Adrenergic uptake inhibitors for ADHD
Medically reviewed by A Ras MD.
What is Qelbree?
Qelbree is a prescription medicine used to treat attention deficit hyperactivity disorder (ADHD) in children 6 to 17 years of age.
It is not known if Qelbree is safe and effective in children less than 6 years of age.
Description
Qelbree contains viloxazine, a selective norepinephrine reuptake inhibitor, in the form of viloxazine hydrochloride which is (±)-2-[(2-ethoxyphenoxy)methyl]morpholine hydrochloride. The molecular formula is C 13H 20NO 3Cl and its molecular weight is 273.8 (HCl salt) with the following structural formula:
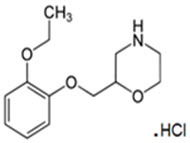
Viloxazine hydrochloride is a white to off-white powder. Viloxazine hydrochloride is soluble in water, 0.1N HCl and aqueous solutions of pH 9.5 and lower. Viloxazine hydrochloride is sparingly soluble in methanol, very slightly soluble in acetonitrile, acetic acid and isopropyl alcohol, and practically insoluble in ethyl acetate.
Qelbree extended-release capsules are intended for oral administration. Each extended-release capsule contains 100 mg, 150 mg, and 200 mg of viloxazine free base equivalent to 115mg, 173mg, and 231mg, respectively, of viloxazine hydrochloride salt.
The inactive ingredients are: Ammonium hydroxide, black iron oxide, butyl alcohol, corn starch, ethylcellulose, FD&C Blue #1, FD&C Red #28, FD&C Yellow #5, FD&C Yellow #6, FD&C Yellow #10, gelatin, hypromellose, isopropyl alcohol, lactose monohydrate, medium chain triglycerides, oleic acid, polyethylene glycol, potassium hydroxide, propylene glycol, shellac, strong ammonia solution, sucrose, talc, triacetin, titanium dioxide
Mechanism of Action
The mechanism of action of viloxazine in the treatment of ADHD is unclear; however, it is thought to be through inhibiting the reuptake of norepinephrine.
What is the most important information I should know about Qelbree?
Qelbree can cause serious side effects, including:
- Increased risk of suicidal thoughts or actions. Qelbree may increase suicidal thoughts and actions in some children with attention deficit hyperactivity disorder (ADHD), especially within the first few months of treatment or when the dose is changed.
How can I watch for and try to prevent suicidal thoughts and actions?- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings or if your child develops suicidal thoughts or actions. This is very important when Qelbree treatment is started or when the dose is changed.
- Call your healthcare provider right away if your child has any new or sudden changes in mood, behavior, thoughts, or feelings, or if your child develops suicidal thoughts or actions.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call your healthcare provider or get emergency help right away if your child has any of the following symptoms, especially if they are new, worse, or worry you:
- attempts to commit suicide
- new or worse depression
- feeling very agitated or restless
- trouble sleeping (insomnia)
- acting aggressive, being angry, or violent
- an extreme increase in activity and talking (mania)
- thoughts about suicide or dying
- new or worse anxiety
- panic attacks
- new or worse irritability
- acting on dangerous impulses
- other unusual changes in behavior or mood
See ” What are the possible side effects of Qelbree?” for more information about side effects.
Who should not take Qelbree?
Do not take Qelbree if your child:
- takes a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if your child takes an MAOI.
- stopped taking an MAOI in the last 14 days.
- takes alosetron, duloxetine, ramelteon, tasimelteon, tizanidine, or theophylline.
What should I tell my healthcare provider before taking Qelbree?
Before taking Qelbree, tell your healthcare provider about all your child’s medical conditions, including if your child:
- has, or has a family history of, suicide, bipolar disorder, depression, mania or hypomania
- has blood pressure or heart rate problems
- has severe kidney problems. Your healthcare provider may lower the dose of Qelbree.
- has liver problems
- is pregnant or plans to become pregnant. Qelbree may cause harm to the mother when taken during pregnancy. You and your healthcare provider will decide if Qelbree should be taken during pregnancy.
- Tell your healthcare provider right away if your child becomes pregnant or thinks they are pregnant during treatment with Qelbree.
- There is a pregnancy registry for females who are exposed to Qelbree during pregnancy. The purpose of the registry is to collect information about the health of females exposed to Qelbree and their baby. If you become pregnant while taking Qelbree, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychiatric Medications by calling 1-866-961-2388 or go to www.womensmentalhealth.org/preg.
- is breastfeeding or plans to breastfeed. It is not known if Qelbree passes into breastmilk. Talk to your healthcare provider about the best way to feed the baby during treatment with Qelbree.
Tell your healthcare provider about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Qelbree and other medicines may affect each other causing possible serious side effects.
Your healthcare provider will decide if Qelbree can be taken with other medicines.
Especially tell your healthcare provider if your child takes:
- MAOIs
- alosetron
- duloxetine
- ramelteon
- tasimelteon
- tizanidine
- theophylline
Know the medicines your child takes. Keep a list of them and show it to your healthcare provider and pharmacist when your child gets a new medicine.
Do not start any new medicine during treatment with Qelbree without first talking to your healthcare provider.
How should I take Qelbree?
- Take Qelbree exactly as your healthcare provider tells you to take it.
- Take Qelbree 1 time each day with or without food.
- Swallow Qelbree capsules whole. Do not cut, crush, or chew the capsules.
- If Qelbree capsules cannot be swallowed whole, the capsule may be opened and the entire contents sprinkled onto a teaspoonful of applesauce.
- Swallow all the applesauce mixture right away, without chewing, or within 2 hours of mixing.
- Do not chew the applesauce mixture.
- Do not store applesauce mixture.
- Talk to your healthcare provider about what you should do if your child misses a dose.
- If you or your child takes too much Qelbree or overdoses, call your poison control center at 1-800-222-1222 right away, or go to the nearest emergency room.
What should I avoid while taking Qelbree?
Do not drive or operate heavy machinery until you know how Qelbree will affect you. Qelbree may cause you to feel sleepy or tired.
What are the possible side effects of Qelbree?
Qelbree can cause serious side effects, including:
- See ” What is the most important information I should know about Qelbree?”
- Increased blood pressure and heart rate. Your healthcare provider should check your child’s blood pressure and heart rate before starting and during treatment with Qelbree.
- Manic episodes. Manic episodes may happen in people with bipolar disorder who take Qelbree. Symptoms may include:
- greatly increased energy
- racing thoughts
- unusually grand ideas
- talking more or faster than usual
- severe trouble sleeping
- reckless behavior
- excessive happiness or irritability
- Sleepiness and tiredness. See ” What should I avoid while taking Qelbree?”
The most common side effects of Qelbree include:
- sleepiness
- tiredness
- vomiting
- irritability
- decreased appetite
- nausea
- trouble sleeping
Effects on weight. Your healthcare provider should check your child’s weight before starting and during treatment with Qelbree.
These are not all of the possible side effects of Qelbree.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Qelbree
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take Qelbree for a condition for which it was not prescribed. Do not give Qelbree to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Qelbree that is written for health professionals.
How should I store Qelbree?
- Store Qelbree capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Qelbree and all medicines out of the reach of children.
What are the ingredients in Qelbree?
Active ingredient: viloxazine
Inactive ingredients: ammonium hydroxide, black iron oxide, butyl alcohol, corn starch, ethylcellulose, FD&C Blue #1, FD&C Red #28, FD&C Yellow #5, FD&C Yellow #6, FD&C Yellow #10, gelatin, hypromellose, isopropyl alcohol, lactose monohydrate, medium-chain triglycerides, oleic acid, polyethylene glycol, potassium hydroxide, propylene glycol, shellac, strong ammonia solution, sucrose, talc, triacetin, titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 100 MG CAPSULE BOTTLE LABEL
- 30 Capsules
NDC 17772-131-30 - Qelbree ™
(viloxazine
extended-release capsules) - 100 mg
- Once daily
For oral use - ATTENTION PHARMACIST:
Dispense the Accompanying
Medication Guide to Each Patient - Rx only


PRINCIPAL DISPLAY PANEL – 150 MG CAPSULE BOTTLE LABEL
- 30 Capsules
NDC 17772-132-30 - Qelbree ™
(viloxazine
extended-release capsules) - 150 mg
- Once daily
For oral use - ATTENTION PHARMACIST:
Dispense the Accompanying
Medication Guide to Each Patient - Rx only


PRINCIPAL DISPLAY PANEL – 200 MG CAPSULE BOTTLE LABEL
- 30 Capsules
NDC 17772-133-30 - Qelbree ™
(viloxazine
extended-release capsules) - 200 mg
- Once daily
For oral use - ATTENTION PHARMACIST:
Dispense the Accompanying
Medication Guide to
Each Patient - Rx only

SRC: NLM .