Rinvoq
Generic name: upadacitinib
Drug class: Antirheumatics
Medically reviewed by A Ras MD.
What is Rinvoq?
Rinvoq is a prescription medicine that is a Janus kinase (JAK) inhibitor. Rinvoq is used to treat adults with moderate to severe rheumatoid arthritis in whom methotrexate did not work well or could not be tolerated.
It is not known if Rinvoq is safe and effective in children under 18 years of age.
Description
RINVOQ is formulated with upadacitinib, a JAK inhibitor.
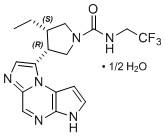
Upadacitinib has the following chemical name: (3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide hydrate (2:1).
The strength of upadacitinib is based on anhydrous upadacitinib. The solubility of upadacitinib in water is 38 to less than 0.2 mg/mL across a pH range of 2 to 9 at 37 oC.
Upadacitinib has a molecular weight of 389.38 g/mol and a molecular formula of C17H19F3N6O • ½ H2O. The chemical structure of upadacitinib is:
RINVOQ 15 mg extended-release tablets for oral administration are purple, biconvex oblong, with dimensions of 14 x 8 mm, and debossed with ‘a15’ on one side. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, ferrosoferric oxide, hypromellose, iron oxide red, magnesium stearate, mannitol, microcrystalline cellulose, polyvinyl alcohol, polyethylene glycol, talc, tartaric acid and titanium dioxide.
RINVOQ 30 mg extended-release tablets for oral administration are red, biconvex oblong, with dimensions of 14 x 8 mm, and debossed with ‘a30’ on one side. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, iron oxide red, magnesium stearate, mannitol, microcrystalline cellulose, polyvinyl alcohol, polyethylene glycol, talc, tartaric acid and titanium dioxide.
RINVOQ 45 mg extended-release tablets for oral administration are yellow to mottled yellow, biconvex oblong, with dimensions of 14 x 8 mm, and debossed with ‘a45’ on one side. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, iron oxide yellow, iron oxide red, magnesium stearate, mannitol, microcrystalline cellulose, polyvinyl alcohol, polyethylene glycol, talc, tartaric acid and titanium dioxide.
Mechanism of Action
Upadacitinib is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of hematopoiesis and immune cell function. Within the signaling pathway, JAKs phosphorylate and activate signal transducers and activators of transcription (STATs) which modulate intracellular activity including gene expression. Upadacitinib modulates the signaling pathway at the point of JAKs, preventing the phosphorylation and activation of STATs.
JAK enzymes transmit cytokine signaling through their pairing (e.g., JAK1/JAK2, JAK1/JAK3, JAK1/TYK2, JAK2/JAK2, JAK2/TYK2). In a cell-free isolated enzyme assay, upadacitinib had greater inhibitory potency at JAK1 and JAK2 relative to JAK3 and TYK2. In human leukocyte cellular assays, upadacitinib inhibited cytokine-induced STAT phosphorylation mediated by JAK1 and JAK1/JAK3 more potently than JAK2/JAK2 mediated STAT phosphorylation. The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.
What is the most important information I should know about Rinvoq?
1. Serious Infections.
Rinvoq is a medicine that affects your immune system. Rinvoq can lower the ability of your immune system to fight infections. Some people have had serious infections while taking Rinvoq, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections.
- Your healthcare provider should test you for TB before starting treatment with Rinvoq.
- Your healthcare provider should watch you closely for signs and symptoms of TB during treatment with Rinvoq.
- You should not start taking Rinvoq if you have any kind of infection unless your healthcare provider tells you it is okay. You may be at a higher risk of developing shingles (herpes zoster).
- Before starting Rinvoq, tell your healthcare provider if you:
- are being treated for an infection.
- have had an infection that does not go away or that keeps coming back.
- have diabetes, chronic lung disease, HIV, or a weak immune system.
- have TB or have been in close contact with someone with TB.
- have had shingles (herpes zoster).
- have had hepatitis B or C.
- live or have lived, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance for getting certain kinds of fungal infections. These infections may happen or become more severe if you use Rinvoq. Ask your healthcare provider if you do not know if you have lived in an area where these infections are common.
- think you have an infection or have symptoms of an infection such as:
- fever, sweating, or chills
- shortness of breath
- warm, red, or painful skin or sores on your body
- muscle aches
- feeling tired
- blood in your phlegm
- diarrhea or stomach pain
- cough
- weight loss
- burning when you urinate or urinating more often than usual
After starting Rinvoq, call your healthcare provider right away if you have any symptoms of an infection. Rinvoq can make you more likely to get infections or make worse any infections that you have.
2. Cancer.
Rinvoq may increase your risk of certain cancers by changing the way your immune system works.
Lymphoma and other cancers, including skin cancers can happen in people taking Rinvoq. Tell your healthcare provider if you have ever had any type of cancer.
3. Blood Clots (thrombosis).
Blood clots in the veins of your legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) and arteries (arterial thrombosis) can happen in some people taking Rinvoq. This may be life-threatening and cause death.
- Tell your healthcare provider if you have had blood clots in the veins of your legs or lungs in the past.
- Tell your healthcare provider right away if you have any signs and symptoms of blood clots during treatment with Rinvoq, including:
- swelling
- pain or tenderness in the leg
- sudden unexplained chest pain
- shortness of breath
4. Tears (perforation) in the stomach or intestines.
- Tell your healthcare provider if you have had diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people taking Rinvoq can get tears in their stomach or intestines. This happens most often in people who take nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or methotrexate.
- Tell your healthcare provider right away if you have fever and stomach-area pain that does not go away, and a change in your bowel habits.
5. Changes in certain laboratory test results.
- Your healthcare provider should do blood tests before you start taking Rinvoq and while you take Rinvoq to check for the following:
- low neutrophil and lymphocyte counts. Neutrophils and lymphocytes are types of white blood cells that help the body fight off infections.
- low red blood cell counts. Red blood cells carry oxygen. Low red blood cells means you may have anemia, which may make you feel weak and tired.
- increased cholesterol levels. Your healthcare provider should do blood tests to check your cholesterol levels approximately 12 weeks after you start taking Rinvoq, and as needed.
- elevated liver enzymes. Liver enzymes help to tell if your liver is functioning normally. Elevated liver enzymes may indicate that your healthcare provider needs to do additional tests on your liver.
You should not take Rinvoq if your neutrophil count, lymphocyte count, or red blood cell count is too low or your liver tests are too high. Your healthcare provider may stop your Rinvoq treatment for a period of time if needed because of changes in these blood test results.
See “What are the possible side effects of Rinvoq?” for more information about side effects
What should I tell my healthcare provider before taking Rinvoq?
Before taking Rinvoq, tell your healthcare provider about all of your medical conditions, including if you:
- See “What is the most important information I should know about Rinvoq?”
- have an infection.
- have liver problems.
- have low red or white blood cell counts.
- have recently received or are scheduled to receive an immunization (vaccine). People who take Rinvoq should not receive live vaccines.
- are pregnant or plan to become pregnant. Based on animal studies, Rinvoq may harm your unborn baby. Your healthcare provider will check whether or not you are pregnant before you start Rinvoq. You should use effective birth control (contraception) to avoid becoming pregnant while taking Rinvoq, and for at least 4 weeks after your last dose of Rinvoq.
- are breastfeeding or plan to breastfeed. Rinvoq may pass into your breast milk. You and your healthcare provider should decide if you will take Rinvoq or breastfeed. You should not do both. You should not breastfeed until 6 days after your last dose of Rinvoq.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Rinvoq and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- medicines for fungal infections (such as ketoconazole, itraconazole, posaconazole or voriconazole) or clarithromycin (for bacterial infections) as these medicines may increase the amount of Rinvoq in your blood.
- rifampicin (for bacterial infections) or phenytoin (for neurological disorders) as these medicines may decrease the effect of Rinvoq.
- medicines that affect your immune system (such as azathioprine and cyclosporine) as these medicines may increase your risk of infection.
Ask your healthcare provider or pharmacist, if you are not sure if you are taking any of these medicines.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Rinvoq?
- Take Rinvoq exactly as your healthcare provider tells you to use it.
- Take Rinvoq 1 time a day with or without food.
- Swallow Rinvoq whole with water at about the same time each day. Do not split or break, crush, or chew the tablets.
What are the possible side effects of Rinvoq?
Rinvoq can cause serious side effects including:
See “What is the most important information I should know about Rinvoq?”
Common side effects of Rinvoq include: upper respiratory tract infections (common cold, sinus infections), nausea, cough, fever.
These are not all the possible side effects of Rinvoq. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Rinvoq
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Rinvoq for a condition for which it was not prescribed.
Do not give Rinvoq to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Rinvoq that is written for health professionals.
How should I store Rinvoq?
- Store Rinvoq in original container at 36°F to 77°F (2°C to 25°C) to protect it from moisture.
- Keep Rinvoq and all medicines out of the reach of children.
What are the ingredients in Rinvoq?
Active ingredient: upadacitinib
Inactive ingredients: microcrystalline cellulose, hypromellose, mannitol, tartaric acid, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, ferrosoferric oxide, and iron oxide red.
Label
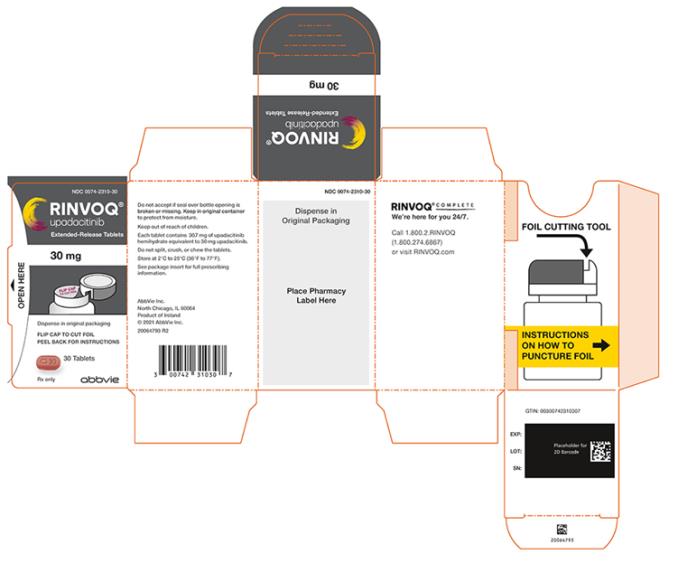
PRINCIPAL DISPLAY PANEL
- NDC 0074-2310-30
- RINVOQ®
- upadacitinib
- Extended-Release Tablets
- 30 mg
- Dispense in original packaging
- FLIP CAP TO CUT FOIL
- PEEL BACK FOR INSTRUCTIONS
- 30 Tablets
- Rx only
- abbvie
PRINCIPAL DISPLAY PANEL
- NDC 0074-1043-28
- RINVOQ®
- upadacitinib
- Extended-Release Tablets
- 45 mg
- Dispense with Medication Guide
- FLIP CAP TO CUT FOIL
- PEEL BACK FOR INSTRUCTIONS
- 28 Tablets
- Rx only
- abbvie
SRC: NLM .