Sogroya
Generic name: somapacitan-beco
Dosage form: injection, for subcutaneous use
Drug class: Growth hormones
Medically reviewed by A Ras MD.
What is Sogroya?
Sogroya is a prescription medicine that contains human growth hormone, the same growth hormone made by the human body.
Sogroya is given by injection under the skin (subcutaneous) and is used to treat adults who do not make enough growth hormone.
It is not known if Sogroya is safe and effective in children.
Who should not use Sogroya?
Do not use Sogroya if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing (respiratory) problems.
- you have cancer or other tumors.
- you are allergic to somapacitan-beco or any of the ingredients in Sogroya. See the end of this patient information guide for a complete list of ingredients in Sogroya.
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes (diabetic retinopathy).
Description
Somapacitan-beco is a human growth hormone (hGH) analog with a single substitution in the amino acid backbone (L101C) to which an albumin-binding moiety has been attached. The albumin-binding moiety (side-chain) consists of an albumin binder and a hydrophilic spacer attached to position 101 of the protein. The protein part consists of 191 amino acids. Somapacitan-beco is produced in Escherichia coli by recombinant DNA technology. The molecular formula (including the albumin-binding moiety) is C1038H1609N273O319S9 and the molecular weight is 23305.10 g/mol, of which the albumin-binding moiety is 1191.39 g/mol.
Structural Formula:
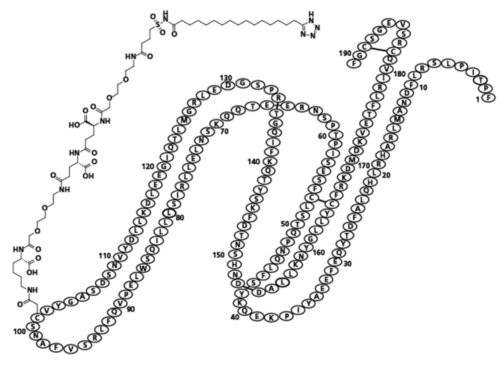
SOGROYA (somapacitan-beco) injection is supplied as a sterile, clear to slightly opalescent and colorless to slightly yellow solution for subcutaneous use in a single-patient-use prefilled pen with a deliverable volume of 1.5 mL.
Each mL of SOGROYA 5 mg/1.5 mL prefilled pen contains 3.3 mg of somapacitan-beco, histidine (0.68 mg), mannitol (44 mg), phenol (4 mg), poloxamer 188 (1 mg), and Water for Injection, USP. The pH is approximately 6.8. Hydrochloric acid and sodium hydroxide may be added to adjust the pH.
Each mL of SOGROYA 10 mg/1.5 mL prefilled pen contains 6.7 mg of somapacitan-beco, histidine (0.68 mg), mannitol (44 mg), phenol (4 mg), poloxamer 188 (1 mg), and Water for Injection, USP. The pH is approximately 6.8. Hydrochloric acid and sodium hydroxide may be added to adjust the pH.
Mechanism of Action
Somapacitan-beco binds to a dimeric GH receptor in the cell membrane of target cells resulting in intracellular signal transduction and a host of pharmacodynamic effects. Some of these pharmacodynamic effects are primarily mediated by insulin-like growth factor 1 (IGF-1) produced in the liver, while others are primarily a consequence of the direct effects of somapacitan-beco.
What should I tell my healthcare provider before using Sogroya?
Before taking Sogroya, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma or serious breathing (respiratory) problems.
- have had cancer or any tumor.
- have diabetes.
- have adrenal gland problems.
- are taking replacement therapy with glucocorticoids.
- have thyroid gland problems.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if Sogroya will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Sogroya passes into your breast milk. You and your healthcare provider should decide if you will take Sogroya while you breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Sogroya may affect how other medicines work, and other medicines may affect how Sogroya works.
How should I use Sogroya?
- Read the detailed Instructions for Use that come with Sogroya.
- Sogroya comes in 1 strength. Your healthcare provider will prescribe the dose that is right for you.
- Your healthcare provider will show you how to inject Sogroya.
- Use Sogroya exactly as your healthcare provider tells you to.
- Use Sogroya 1 time each week.
- If you miss a dose of Sogroya, take the missed dose as soon as possible within 3 days (72 hours) after the missed dose. If more than 3 days (72 hours) have passed, skip the missed dose and take your next dose on the regularly scheduled day.
- Sogroya pens are for use by 1 person only.
- Do not share your Sogroya pens and needles with another person, even if the needle has been changed. You may give another person an infection or get an infection from them.
What are the possible side effects of Sogroya?
Sogroya may cause serious side effects, including:
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma or serious breathing (respiratory) problems.
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer. Your healthcare provider will need to monitor you for a return of cancer or a tumor. Contact the healthcare provider if you start to have changes in moles, birthmarks, or the color of your skin.
- new or worsening high blood sugar (hyperglycemia) or diabetes. Your blood sugar may need to be monitored during treatment with Sogroya.
- increase in pressure in the skull (intracranial hypertension). If you have headaches, eye problems, nausea or vomiting, contact the healthcare provider.
- serious allergic reactions. Get medical help right away if you have the following symptoms:
- swelling of your face, lips, mouth, or tongue
- trouble breathing
- wheezing
- skin rashes, redness, or swelling
- dizziness or fainting
- fast heartbeat or pounding in your chest
- severe itching
- sweating
- your body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your joints or muscles or nerve problems that cause pain, burning or tingling in the hands, arms, legs and feet. Tell your healthcare provider if you have any of these signs or symptoms of fluid retention.
- decrease in a hormone called cortisol. The healthcare provider will do blood tests to check your cortisol levels. Tell your healthcare provider if you have darkening of the skin, severe fatigue, dizziness, weakness, or weight loss.
- decrease in thyroid hormone levels. Decreased thyroid hormone levels may affect how well Sogroya works. The healthcare provider will do blood tests to check your thyroid hormone levels.
- severe and constant abdominal pain. This could be a sign of pancreatitis. Tell your healthcare provider if you have any new abdominal pain.
- loss of fat and tissue weakness in the area of skin you inject. Talk to your healthcare provider about rotating the areas where you inject Sogroya.
- increase in phosphorus, alkaline phosphatase and parathyroid hormone levels in your blood. Your healthcare provider will do blood tests to check this.
The most common side effects of Sogroya include:
- back pain
- joint pain
- indigestion
- sleep problems
- dizziness
- swelling of the tonsils (tonsillitis)
- vomiting
- high blood pressure
- increase in the level of an enzyme in your blood called creatine phosphokinase
- weight gain
- low red blood cells (anemia)
These are not all the possible side effects of Sogroya.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Novo Nordisk at 1-888-668-6444.
General information about the safe and effective use of Sogroya
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Sogroya for a condition for which it was not prescribed. Do not give Sogroya to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Sogroya that is written for health professionals.
How should I store Sogroya?
- Before you use Sogroya pens for the first time:
- Store your new, unused Sogroya pen in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- Store your new, unused Sogroya pen with the cap on and keep it in the original carton.
- Do not freeze Sogroya.
- Keep Sogroya away from direct heat and light.
- Do not use Sogroya that has been frozen or in temperatures warmer than 86ºF (30ºC).
- Do not use Sogroya after the expiration date printed on the carton and the pen.
- After you use Sogroya pens and there is still medicine left:
- Store remaining Sogroya in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) and use within 6 weeks.
- Store your in-use Sogroya pen with the cap on and keep it in the original carton.
- If needed, unused and in-use Sogroya pens can be stored out of the refrigerator. Sogroya pens can be stored at room temperature no warmer than 77ºF (25ºC) for up to 3 days (72 hours) and then returned to the refrigerator.
Keep Sogroya and all medicines out of the reach of children.
What are the ingredients in Sogroya?
Active ingredient: somapacitan-beco
Inactive ingredients: histidine, mannitol, phenol, poloxamer 188, Water for Injection, and hydrochloric acid and sodium hydroxide (as needed)
Label
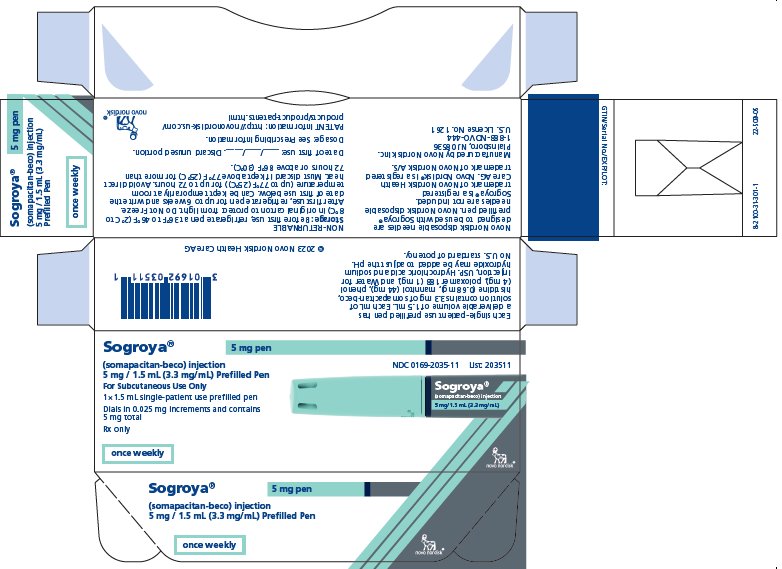
PRINCIPAL DISPLAY PANEL – 5 MG
- Sogroya® 5 mg pen NDC 0169-2035-11
- (somapacitan-beco) injection List: 203511
- 5 mg / 1.5 mL (3.3 mg/mL) Prefilled Pen
- For subcutaneous Use Only
- 1×1.5 mL single-patient use prefilled pen
- Dials in 0.025 mg increments and contains 5 mg total
- Rx only
- once weekly
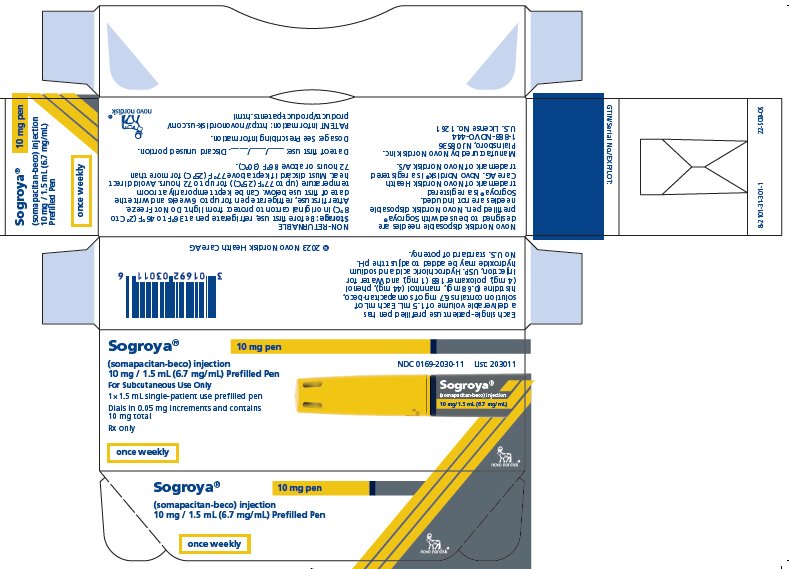
PRINCIPAL DISPLAY PANEL – 10 MG
- Sogroya® 10 mg pen NDC 0169-2030-11
- (somapacitan-beco) injection List: 203011
- 10 mg / 1.5 mL (6.7 mg/mL) Prefilled Pen
- For subcutaneous Use Only
- 1×1.5 mL single-patient use prefilled pen
- Dials in 0.05 mg increments and contains 10 mg total
- Rx only
- once weekly
SRC: NLM .