Synjardy XR
Generic name: empagliflozin and metformin
Brand names: Synjardy, Synjardy XR
Drug class: Antidiabetic combinations
Medically reviewed by A Ras MD.
What is Synjardy XR?
Synjardy XR is a prescription medicine that contains 2 prescription diabetes medicines, empagliflozin and metformin. Synjardy XR can be used along with diet and exercise to improve blood sugar in adults with type 2 diabetes, in adults with type 2 diabetes who have known cardiovascular disease when both empagliflozin and metformin is appropriate and empagliflozin is needed to reduce the risk of cardiovascular death.
Synjardy XR is not for people with type 1 diabetes. Synjardy XR is not for people with diabetic ketoacidosis (increased ketones in the blood or urine). It is not known if Synjardy XR is safe and effective in children under 18 years of age.
Description
SYNJARDY XR tablets for oral use contain: empagliflozin and metformin hydrochloride.
Empagliflozin
Empagliflozin is an inhibitor of the sodium-glucose co-transporter 2 (SGLT2).
The chemical name of empagliflozin is D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-, (1S).
Its molecular formula is C23H27ClO7 and the molecular weight is 450.91. The structural formula is:
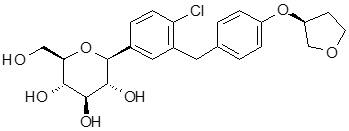
Empagliflozin is a white to yellowish, non-hygroscopic powder. It is very slightly soluble in water, sparingly soluble in methanol, slightly soluble in ethanol and acetonitrile; soluble in 50% acetonitrile/water; and practically insoluble in toluene.
Metformin hydrochloride
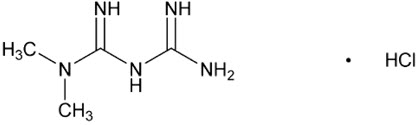
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is a biguanide. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5∙HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
Each film-coated tablet of SYNJARDY XR consists of an extended-release metformin hydrochloride core tablet that is coated with the immediate-release drug substance empagliflozin.
SYNJARDY XR tablets for oral administration are available in four dosage strengths containing:
- 5 mg empagliflozin and 1000 mg metformin hydrochloride extended-release
- 10 mg empagliflozin and 1000 mg metformin hydrochloride extended-release
- 12.5 mg empagliflozin and 1000 mg metformin hydrochloride extended-release
- 25 mg empagliflozin and 1000 mg metformin hydrochloride extended-release
Each film-coated tablet of SYNJARDY XR contains the following inactive ingredients: Tablet Core: polyethylene oxide, hypromellose, and magnesium stearate. Film Coatings and Printing Ink: hypromellose, titanium dioxide, polydextrose, polyethylene glycol, talc, carnauba wax, purified water, ferrosoferric oxide, propylene glycol, isopropyl alcohol, ferric oxide yellow (5 mg/1000 mg, 10 mg/1000 mg, 25 mg/1000 mg), ferric oxide red (10 mg/1000 mg), FD&C blue#2/indigo carmine aluminum lake (12.5 mg/1000 mg, 25 mg/1000 mg).
Mechanism of Action
SYNJARDY XR
SYNJARDY XR contains: empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, and metformin, a biguanide.
Empagliflozin
Sodium-glucose co-transporter 2 (SGLT2) is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. Empagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Metformin HCl
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. It is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike SUs, metformin does not produce hypoglycemia in either patients with type 2 diabetes mellitus or normal subjects (except in special circumstances) and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
What is the most important information I should know about Synjardy XR?
Serious side effects can happen in people taking Synjardy XR, including:
Lactic Acidosis. Metformin, one of the medicines in Synjardy XR can cause a rare but serious condition called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Call your doctor right away if you have any of the following symptoms, which could be signs of lactic acidosis:
- you feel cold in your hands or feet
- you feel dizzy or lightheaded
- you have a slow or irregular heartbeat
- you feel very weak or tired
- you have unusual (not normal) muscle pain
- you have trouble breathing
- you feel sleepy or drowsy
- you have stomach pains, nausea or vomiting
Most people who have had lactic acidosis with metformin have other things that, combined with metformin, led to the lactic acidosis. Tell your doctor if you have any of the following, because you have a higher chance for getting lactic acidosis with Synjardy XR if you:
- have moderate to severe kidney problems or your kidneys are affected by certain x-ray tests that use injectable dye.
- have liver problems
- drink alcohol very often, or drink a lot of alcohol in the short-term (“binge” drinking)
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have surgery
- have a heart attack, severe infection, or stroke
The best way to keep from having a problem with lactic acidosis from metformin is to tell your doctor if you have any of the problems in the list above. Your doctor may decide to stop your Synjardy XR for a while if you have any of these things.
Synjardy XR can have other serious side effects. See “What are the possible side effects of Synjardy XR?”
Who should not take Synjardy XR?
Do not take Synjardy XR if you:
- have moderate to severe kidney problems or are on dialysis
- have a condition called metabolic acidosis or diabetic ketoacidosis (increased ketones in the blood or urine)
- are allergic to empagliflozin, metformin, or any of the ingredients in Synjardy XR. See the end of this Medication Guide for a list of ingredients in Synjardy XR.
What should I tell my healthcare provider before taking Synjardy XR?
Before taking Synjardy XR, tell your healthcare provider about all of your medical conditions, including if you:
- have moderate to severe kidney problems
- have liver problems
- have a history of urinary tract infection or problems with urination
- have heart problems, including congestive heart failure
- are going to have surgery. Your doctor may stop your Synjardy XR before you have surgery. Talk to your doctor if you are having surgery about when to stop taking Synjardy XR and when to start it again.
- are eating less, or there is a change in your diet
- have or have had problems with your pancreas, including pancreatitis or surgery on your pancreas
- drink alcohol very often, or drink a lot of alcohol in the short term (“binge” drinking)
- are going to get an injection of dye or contrast agents for an x-ray procedure. Synjardy XR may need to be stopped for a short time. Talk to your doctor about when you should stop Synjardy XR and when you should start Synjardy XR again. See “What is the most important information I should know about Synjardy XR?”
- have type 1 diabetes. Synjardy XR should not be used to treat people with type 1 diabetes.
- have any other medical conditions
- are pregnant or plan to become pregnant. Synjardy XR may harm your unborn baby. If you become pregnant while taking Synjardy XR, tell your doctor as soon as possible. Talk with your doctor about the best way to control your blood sugar while you are pregnant.
- are a premenopausal woman (before the “change of life”), who does not have periods regularly or at all. Talk to your doctor about birth control choices while taking Synjardy XR if you are not planning to become pregnant since Synjardy XR may increase your chance of becoming pregnant. Tell your doctor right away if you become pregnant while taking Synjardy XR.
- are breastfeeding or plan to breastfeed. Synjardy XR may pass into your breast milk and may harm your baby. Talk with your doctor about the best way to feed your baby if you are taking Synjardy XR. Do not breastfeed while taking Synjardy XR.
Tell your healthcare provider about all the medicines you take, including prescription or over-the-counter medicines, vitamins, or herbal supplements.
How should I take Synjardy XR?
- Take Synjardy XR exactly as your doctor tells you to take it.
- Take Synjardy XR by mouth 1 time each day with a meal in the morning. Taking Synjardy XR with a meal may lower your chance of having an upset stomach.
- Take Synjardy XR tablets whole. Do not break, cut, crush, dissolve, or chew Synjardy XR tablets before swallowing. If you cannot swallow Synjardy XR tablets whole, tell your doctor.
- You may see something that looks like the Synjardy XR tablet in your stool (bowel movement). If you see tablets in your stool talk to your doctor. Do not stop taking Synjardy XR without talking to your doctor.
- Your doctor will tell you how much Synjardy XR to take and when to take it.
- Your doctor may change your dose if needed.
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take two doses of Synjardy XR at the same time. Talk with your doctor if you have questions about a missed dose.
- Your doctor may tell you to take Synjardy XR along with other diabetes medicines. Low blood sugar can happen more often when Synjardy XR is taken with certain other diabetes medicines. See “What are the possible side effects of Synjardy XR?”
- If you take too much Synjardy XR, call your doctor or go to the nearest hospital emergency room right away.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your doctor right away if you have any of these conditions and follow your doctor’s instructions.
- Check your blood sugar as your doctor tells you to.
- When taking Synjardy XR, you may have sugar in your urine, which will show up on a urine test.
- Stay on your prescribed diet and exercise program while taking Synjardy XR.
- Talk to your doctor about how to prevent, recognize and manage low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and complications of diabetes.
- Your doctor will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Your doctor will do blood tests to check how well your kidneys are working before and during your treatment with Synjardy XR.
- Your doctor may do certain blood tests before you start Synjardy XR and during treatment.
What should I avoid while taking Synjardy XR?
Avoid drinking alcohol very often, or drinking a lot of alcohol in a short period of time (“binge” drinking). It can increase your chances of getting serious side effects.
What are the possible side effects of Synjardy XR?
Synjardy XR may cause serious side effects, including:
- See “What is the most important information I should know about Synjardy XR?”
- Dehydration. Synjardy XR can cause some people to have dehydration (the loss of body water and salt). Dehydration may cause you to feel dizzy, faint, light-headed, or weak, especially when you stand up (orthostatic hypotension). You may be at higher risk of dehydration if you:
- have low blood pressure
- have kidney problems
- are 65 years of age or older
- are on low sodium (salt) diet
- take medicines to lower your blood pressure, including diuretics (water pills)
- Ketoacidosis (increased ketones in your blood or urine). Ketoacidosis has happened in people who have type 1 diabetes or type 2 diabetes, during treatment with empagliflozin, one of the medicines in Synjardy XR. Ketoacidosis has also happened in people with diabetes who were sick or who had surgery during treatment with Synjardy XR. Ketoacidosis is a serious condition, which may need to be treated in a hospital. Ketoacidosis may lead to death. Ketoacidosis can happen with Synjardy XR even if your blood sugar is less than 250 mg/dL. Stop taking Synjardy XR and call your doctor right away if you get any of the following symptoms:
- nausea
- vomiting
- stomach-area (abdominal) pain
- tiredness
- trouble breathing
If you get any of these symptoms during treatment with Synjardy XR, if possible, check for ketones in your urine, even if your blood sugar is less than 250 mg/dL.
- Kidney problems. Sudden kidney injury has happened to people taking Synjardy XR. Talk to your doctor right away if you:
- reduce the amount of food or liquid you drink for example, if you are sick or cannot eat or
- start to lose liquids from your body for example, from vomiting, diarrhea or being in the sun too long
- Serious urinary tract infections. Serious urinary tract infections that may lead to hospitalization have happened in people who are taking empagliflozin, one of the medicines in Synjardy XR. Tell your doctor if you have any signs or symptoms of a urinary tract infection such as a burning feeling when passing urine, a need to urinate often, the need to urinate right away, pain in the lower part of your stomach (pelvis), or blood in the urine. Sometimes people also may have a fever, back pain, nausea or vomiting.
- Low blood sugar (hypoglycemia). If you take Synjardy XR with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take Synjardy XR. Signs and symptoms of low blood sugar may include:
- headache
- drowsiness
- weakness
- irritability
- hunger
- fast heartbeat
- confusion
- shaking or feeling jittery
- dizziness
- sweating
- A rare but serious bacterial infection that causes damage to the tissue under the skin (necrotizing fasciitis) in the area between and around the anus and genitals (perineum). Necrotizing fasciitis of the perineum has happened in women and men who take empagliflozin, one of the medicines in Synjardy XR. Necrotizing fasciitis of the perineum may lead to hospitalization, may require multiple surgeries, and may lead to death. Seek medical attention immediately if you have a fever or you are feeling very weak, tired or uncomfortable (malaise), and you develop any of the following symptoms in the area between and around your anus and genitals:
- pain or tenderness
- swelling
- redness of skin (erythema)
- Vaginal yeast infection. Women who take Synjardy XR may get vaginal yeast infections. Symptoms of a vaginal yeast infection include vaginal odor, white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese), or vaginal itching.
- Yeast infection of the penis (balanitis). Men who take Synjardy XR may get a yeast infection of the skin around the penis. Certain men who are not circumcised may have swelling of the penis that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of yeast infection of the penis include redness, itching, or swelling of the penis, rash of the penis, foul smelling discharge from the penis, or pain in the skin around the penis.
Talk to your doctor about what to do if you get symptoms of a yeast infection of the vagina or penis. Your doctor may suggest you use an over-the-counter antifungal medicine. Talk to your doctor right away if you use an over-the-counter antifungal medication and your symptoms do not go away. - Allergic (hypersensitivity) reactions. Serious allergic reactions have happened in people who are taking empagliflozin, one of the medicines in Synjardy XR. Symptoms may include
If you have any of these symptoms, stop taking Synjardy XR and call your doctor right away or go to the nearest hospital emergency room.- swelling of your face, lips, throat and other areas of your skin
- difficulty with swallowing or breathing.
- raised, red areas on your skin (hives)
- Low vitamin B12 (vitamin B12 deficiency). Using metformin for long periods of time may cause a decrease in the amount of vitamin B12 in your blood, especially if you have had low vitamin B12 blood levels before. Your doctor may do blood tests to check your vitamin B12 levels.
- Increased fats in your blood (cholesterol)
Other common side effects of Synjardy XR include diarrhea, nausea, vomiting, gas, stomach pain, indigestion, weakness, and headache.
These are not all the possible side effects of Synjardy XR. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Synjardy XR
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Synjardy XR for a condition for which it was not prescribed. Do not give Synjardy XR to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Synjardy XR. If you would like more information, talk with your doctor. You can ask your pharmacist or healthcare provider for information about Synjardy XR that is written for health professionals.
How should I store Synjardy XR?
Store Synjardy XR at room temperature between 68°F to 77°F (20°C to 25°C).
What are the ingredients in Synjardy XR?
Active Ingredients: empagliflozin and metformin hydrochloride
Inactive Ingredients:
Tablet core contains: polyethylene oxide, hypromellose, and magnesium stearate.
The Film Coatings and Printing Ink contain: hypromellose, titanium dioxide, polydextrose, polyethylene glycol, talc, carnauba wax, purified water, ferrosoferric oxide, propylene glycol, isopropyl alcohol, ferric oxide yellow (5 mg/1000 mg, 10 mg/1000 mg, 25 mg/1000 mg), ferric oxide red (10 mg/1000 mg), FD&C blue#2/indigo carmine aluminum lake (12.5 mg/1000 mg, 25 mg/1000 mg).
Label
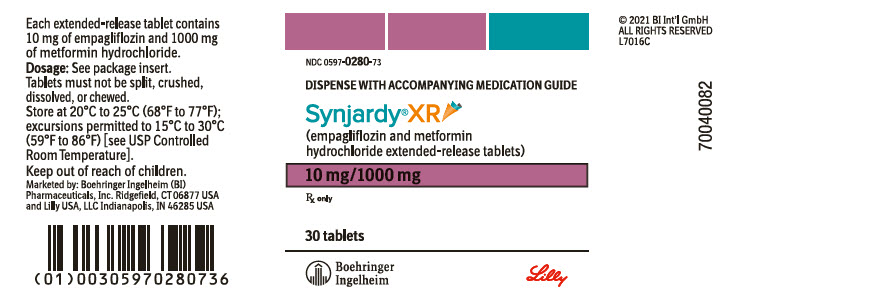
PRINCIPAL DISPLAY PANEL – 10 MG/1000 MG TABLET BOTTLE LABEL
- NDC 0597-0280-73
- DISPENSE WITH ACCOMPANYING MEDICATION GUIDE
- Synjardy®XR
(empagliflozin and metformin
hydrochloride extended-release tablets) - 10 mg/1000 mg
- Rx only
- 30 tablets
- Boehringer
Ingelheim - Lilly
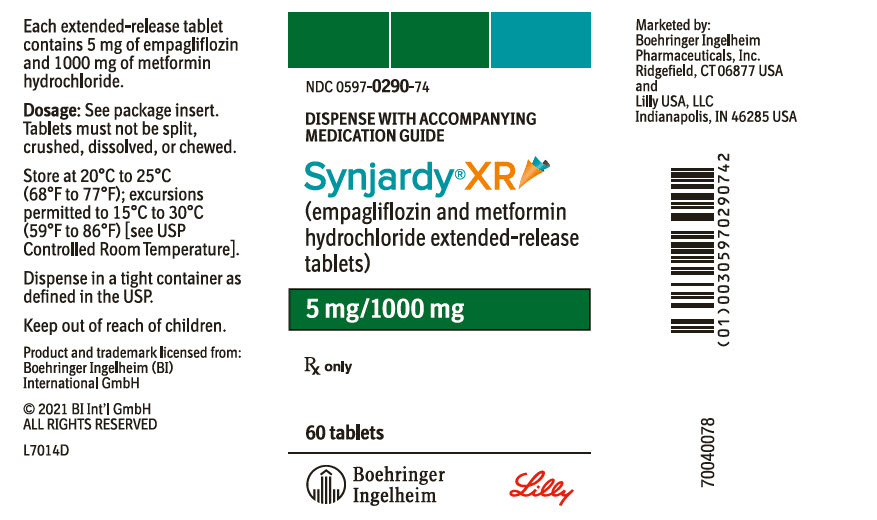
PRINCIPAL DISPLAY PANEL – 5 MG/1000 MG TABLET BOTTLE LABEL
- NDC 0597-0290-74
- DISPENSE WITH ACCOMPANYING
MEDICATION GUIDE - Synjardy®XR
(empagliflozin and metformin
hydrochloride extended-release
tablets) - 5 mg/1000 mg
- Rx only
- 60 tablets
- Boehringer
Ingelheim - Lilly
PRINCIPAL DISPLAY PANEL – 25 MG/1000 MG TABLET BOTTLE LABEL
- NDC 0597-0295-88
- DISPENSE WITH ACCOMPANYING MEDICATION GUIDE
- Synjardy®XR
(empagliflozin and metformin
hydrochloride extended-release tablets) - 25 mg/1000 mg
- Rx only
- 30 tablets
- Boehringer
Ingelheim - Lilly
PRINCIPAL DISPLAY PANEL – 12.5 MG/1000 MG TABLET BOTTLE LABEL
- NDC 0597-0300-45
- DISPENSE WITH ACCOMPANYING
MEDICATION GUIDE - Synjardy®XR
(empagliflozin and metformin
hydrochloride extended-release
tablets) - 12.5 mg/1000 mg
- Rx only
- 60 tablets
- Boehringer
Ingelheim - Lilly

SRC: NLM .


