Tagrisso
Generic name: osimertinib
Drug class: EGFR inhibitors
Medically reviewed by A Ras MD.
What is Tagrisso?
Tagrisso is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC) that has certain abnormal epidermal growth factor receptor (EGFR) gene(s):
- to help prevent your lung cancer from coming back after your tumor(s) has been removed by surgery, or
- as your first treatment when your lung cancer has spread to other parts of the body (metastatic), or
- when your lung cancer has spread to other parts of the body (metastatic) and you have had previous treatment with an EGFR tyrosine kinase inhibitor (TKI) medicine that did not work or is no longer working.
Your healthcare provider will perform a test to make sure that Tagrisso is right for you.
It is not known if Tagrisso is safe and effective in children.
Description
Osimertinib is a kinase inhibitor for oral use. The molecular formula for osimertinib mesylate is C28H33N7O2•CH4O3S, and the molecular weight is 596 g/mol. The chemical name is N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide mesylate salt. Osimertinib has the following structural formula (as osimertinib mesylate):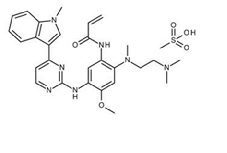
TAGRISSO tablets contain 40 or 80 mg of osimertinib, equivalent to 47.7 and 95.4 mg of osimertinib mesylate, respectively. Inactive ingredients in the tablet core are mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose and sodium stearyl fumarate. The tablet coating consists of polyvinyl alcohol, titanium dioxide, macrogol 3350, talc, ferric oxide yellow, ferric oxide red and ferric oxide black.
Mechanism of Action
Osimertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR), which binds irreversibly to certain mutant forms of EGFR (T790M, L858R, and exon 19 deletions) at approximately 9-fold lower concentrations than wild-type. Two pharmacologically-active metabolites (AZ7550 and AZ5104 circulating at approximately 10% of the parent) with similar inhibitory profiles to osimertinib have been identified in the plasma after oral administration of osimertinib. AZ7550 showed a similar potency to osimertinib, while AZ5104 showed greater potency against exon 19 deletion and T790M mutants (approximately 8-fold) and wild-type (approximately 15-fold) EGFR. In vitro, osimertinib also inhibited the activity of HER2, HER3, HER4, ACK1, and BLK at clinically relevant concentrations.
In cultured cells and animal tumor implantation models, osimertinib exhibited anti-tumor activity against NSCLC lines harboring EGFR-mutations (T790M/L858R, L858R, T790M/exon 19 deletion, and exon 19 deletion) and, to a lesser extent, wild-type EGFR amplifications. Osimertinib distributed to the brain in multiple animal species (monkey, rat, and mouse) with brain to plasma AUC ratios of approximately 2 following oral dosing. These data are consistent with observations of tumor regression and increased survival in osimertinib- versus control-treated animals in a pre-clinical mutant-EGFR intracranial mouse metastasis xenograft model (PC9; exon 19 deletion).
What is the most important information I should know about Tagrisso?
Tagrisso may cause serious side effects, including:
- lung problems. Tagrisso may cause lung problems that may lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you have any new or worsening lung symptoms, including trouble breathing, shortness of breath, cough, or fever.
- heart problems, including heart failure. Tagrisso may cause heart problems that may lead to death. Your healthcare provider should check your heart function before you start taking Tagrisso and during treatment as needed. Tell your healthcare provider right away if you have any of the following signs and symptoms of a heart problem: feeling like your heart is pounding or racing, shortness of breath, swelling of your ankles and feet, feeling lightheaded.
- eye problems. Tagrisso may cause eye problems. Tell your healthcare provider right away if you have symptoms of eye problems which may include watery eyes, sensitivity to light, eye pain, eye redness, or vision changes. Your healthcare provider may send you to see an eye specialist (ophthalmologist) if you get eye problems with Tagrisso.
- skin problems. Tagrisso may cause skin problems. Tell your healthcare provider right away if you develop target lesions (skin reactions that look like rings), severe blistering or peeling of the skin.
- inflammation of the blood vessels in your skin. Tagrisso may cause blood vessel problems in your skin. Tell your healthcare provider right away if you develop purple spots or redness of the skin that does not fade in color when pressed (non-blanching) on your lower arms, lower legs, or buttocks or large hives on the main part of your body (trunk) that do not go away within 24 hours and look bruised.
See “What are the possible side effects of Tagrisso?” for more information about side effects.
What should I tell my healthcare provider before taking Tagrisso?
Before taking Tagrisso, tell your doctor about all of your medical conditions, including if you:
- have lung or breathing problems.
- have heart problems, including a condition called long QTc syndrome.
- have problems with your electrolytes, such as sodium, potassium, calcium or magnesium.
- have a history of eye problems.
- are pregnant or plan to become pregnant. Tagrisso can harm your unborn baby. Tell your doctor right away if you become pregnant during treatment with Tagrisso or think you may be pregnant.
- Females who are able to become pregnant should use effective birth control during treatment with Tagrisso and for 6 weeks after the final dose of Tagrisso.
- Males who have female partners that are able to become pregnant should use effective birth control during treatment with Tagrisso and for 4 months after the final dose of Tagrisso.
- are breastfeeding or plan to breastfeed. It is not known if Tagrisso passes into your breast milk. Do not breastfeed during treatment with Tagrisso and for 2 weeks after your final dose of Tagrisso. Talk to your doctor about the best way to feed your baby during this time.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements. Especially tell your doctor if you take a heart or blood pressure medicine.
How should I take Tagrisso?
- Take Tagrisso exactly as your doctor tells you to take it.
- Your doctor may change your dose, temporarily stop, or permanently stop treatment with Tagrisso if you have side effects.
- Take Tagrisso 1 time each day.
- You can take Tagrisso with or without food.
- If you miss a dose of Tagrisso, do not make up for the missed dose. Take your next dose at your regular time.
- If you cannot swallow Tagrisso tablets whole:
- place your dose of Tagrisso in a container that contains 60 mL (2 ounces) of water. Do not use carbonated water or any other liquids.
- stir the Tagrisso tablet and water until the Tagrisso tablet is in small pieces (the tablet will not completely dissolve). Do not crush, heat, or use ultrasound to prepare the mixture.
- drink the Tagrisso and water mixture right away.
- add 120 mL to 240 mL (4 to 8 ounces) of water into the container and drink to make sure that you take your full dose of Tagrisso.
What are the possible side effects of Tagrisso?
Tagrisso may cause serious side effects, including:
- See “What is the most important information I should know about Tagrisso?”
- Severe blistering or peeling of skin – seek medical attention right away if you develop these symptoms.
- Target lesions, which are skin reactions that look like rings – seek medical attention right away if you develop these symptoms.
The most common side effects of Tagrisso are:
- low white blood cell counts
- low platelet counts
- diarrhea
- nausea
- low red blood cell counts (anemia)
- rash
- muscle, bone, or joint pain
- changes in your nails, including: redness, tenderness, pain, inflammation, brittleness, separation from the nailbed, and shedding of nail
- dry skin mouth
- sores
- tiredness
- cough
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Tagrisso. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tagrisso
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Tagrisso for a condition for which it was not prescribed. Do not give Tagrisso to other people, even if they have the same symptoms you have. It may harm them. You can ask your doctor or pharmacist for information about Tagrisso that is written for a healthcare professional.
How should I store Tagrisso?
- Store Tagrisso at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away medicine that is out of date or that you no longer need.
- Keep Tagrisso and all medicines out of the reach of children.
What are the ingredients in Tagrisso?
Active ingredient: osimertinib
Inactive ingredients: mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and sodium stearyl fumarate.
Tablet coating contains: polyvinyl alcohol, titanium dioxide, macrogol 3350, talc, ferric oxide yellow, ferric oxide red and ferric oxide black.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 40 MG
- NDC 0310-1349-30 30 Tablets
- Tagrisso®
- (osimertinib) tablets
- 40 mg per tablet
- Rx only
- AstraZeneca


PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 80 MG
- NDC 0310-1350-30 30 Tablets
- Tagrisso®
- (osimertinib) tablets
- 80 mg per tablet
- Rx only
- AstraZeneca


SRC: NLM .