Tamiflu
Generic name: oseltamivir
Drug class: Neuraminidase inhibitors
Medically reviewed by A Ras MD
What is Tamiflu?
Tamiflu is a prescription medicine used to treat the flu (influenza) in people 2 weeks of age and older who have had flu symptoms for no more than two days. It prevents the flu in people who are 1 year of age and older.
It is not known if Tamiflu is:
- effective in people who start treatment after 2 days of developing flu symptoms.
- effective for the treatment of the flu in people with long-time (chronic) heart problems or breathing problems.
- effective for the treatment or prevention of flu in people who have weakened immune systems (immunocompromised)
- safe and effective for the treatment of the flu in children less than 2 weeks of age.
- safe and effective in the prevention of the flu in children less than 1 year of age.
Tamiflu does not treat or prevent illness that is caused by infections other than the influenza virus.
Tamiflu does not prevent bacterial infections that may happen with the flu.
Tamiflu is not recommended for people with end-stage renal disease (ESRD) who are not receiving dialysis.
Tamiflu does not take the place of receiving a flu vaccination. Talk to your healthcare provider about when you should receive an annual flu vaccination.
Description
TAMIFLU (oseltamivir phosphate), an influenza neuraminidase inhibitor (NAI), is available as:
- Capsules containing 30 mg, 45 mg, or 75 mg of oseltamivir for oral use, in the form of oseltamivir phosphate, and
- A powder for oral suspension, which when constituted with water as directed contains 6 mg per mL oseltamivir base.
In addition to the active ingredient, each capsule contains croscarmellose sodium, povidone K30, pregelatinized starch, sodium stearyl fumarate and talc. The 30 mg capsule shell contains gelatin, red iron oxide, titanium dioxide, and yellow iron oxide. The 45 mg capsule shell contains black iron oxide, gelatin, and titanium dioxide. The 75 mg capsule shell contains black iron oxide, gelatin, red iron oxide, titanium dioxide, and yellow iron oxide. Each capsule is printed with blue ink, which includes FD&C Blue No. 2 as the colorant.
In addition to the active ingredient, the powder for oral suspension contains monosodium citrate, saccharin sodium, sodium benzoate, sorbitol, titanium dioxide, tutti-frutti flavoring, and xanthan gum.
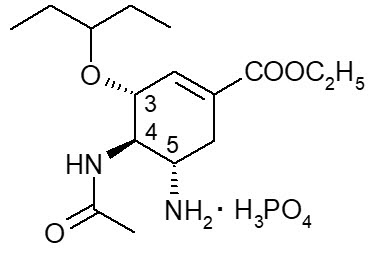
Oseltamivir phosphate is a white crystalline solid with the chemical name (3R,4R,5S)-4-acetylamino-5-amino-3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1). The chemical formula is C16H28N2O4 (free base). The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt. The structural formula is as follows:
Who should not take Tamiflu?
Do not take Tamiflu if you are allergic to oseltamivir phosphate or any of the ingredients in Tamiflu. See below for a complete list of ingredients in Tamiflu.
What should I tell my healthcare provider before taking Tamiflu?
Before you take Tamiflu, tell your healthcare provider if you:
- have problems swallowing Tamiflu capsules
- have kidney problems
- have a history of fructose (fruit sugar) intolerance. Tamiflu contains sorbitol and may cause stomach upset and diarrhea in people who are fructose intolerant.
- have any other medical conditions
- are pregnant or plan to become pregnant. Available information indicate that Tamiflu does not increase the risk of birth defects.
- are breastfeeding or plan to breastfeed. Tamiflu can pass into breast milk in small amounts.
Tell your healthcare provider about all the medicines you take, including prescription or over-the counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Tamiflu?
- Take Tamiflu exactly as your healthcare provider tells you to.
- Take Tamiflu with food or without food. There is less chance of stomach upset if you take Tamiflu with food.
- If you miss a dose of Tamiflu, take it as soon as you remember. If it is 2 hours or less before your next dose, do not take the missed dose. Take your next dose of Tamiflu at your scheduled time. Do not take 2 doses at the same time.
- If Tamiflu for oral suspension is not available or you cannot swallow Tamiflu capsules, your healthcare provider or pharmacist may instruct you to open Tamiflu capsules and mix the capsules contents with sweetened liquids such as chocolate syrup (regular or sugar-free), corn syrup, caramel topping, or light brown sugar (dissolved in water).
- If your healthcare provider or pharmacist has instructed you to take Tamiflu for oral suspension or open your Tamiflu capsules, read the detailed Instructions for Use on the leaflet you receive with your medication. Ask your pharmacist if you have any questions.
What are the possible side effects of Tamiflu?
Tamiflu may cause serious side effects, including:
- Serious skin and allergic reactions. Tamiflu can cause serious skin and allergic reactions. Stop taking Tamiflu and get medical help right away if you get any of the following symptoms:
- skin rash or hives
- your skin blisters and peels
- blisters or sores in your mouth
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing
- itching
- chest pain or tightness
- Change in behavior. People, especially children, who have the flu, can develop nervous system problems and abnormal behavior that can lead to death. During treatment with Tamiflu, tell your healthcare provider right away if you or your child have confusion, speech problems, shaky movements, seizures, or start hearing voices or seeing things that are not really there (hallucinations).
The most common side effects of Tamiflu when used for treatment of the flu include nausea, vomiting, and headache.
The most common side effect of Tamiflu when used for prevention of the flu include, nausea, vomiting, headache, and pain.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Tamiflu.
Label
PRINCIPAL DISPLAY PANEL – 30 MG CAPSULE BLISTER PACK CARTON
- NDC 0004-0802-85
- Tamiflu®
(oseltamivir phosphate) Capsules - 30 mg
- Each capsule contains oseltamivir phosphate equivalent to 30 mg
oseltamivir (free base). - Rx only
- 10 Capsules
- Genentech
- 10175386

PRINCIPAL DISPLAY PANEL – 45 MG CAPSULE BLISTER PACK CARTON
- NDC 0004-0801-85
- Tamiflu®
(oseltamivir phosphate) Capsules - 45 mg
- Each capsule contains oseltamivir phosphate equivalent to 45 mg
oseltamivir (free base). - Rx only
- 10 Capsules
- Genentech
- 10175387

PRINCIPAL DISPLAY PANEL – 75 MG CAPSULE BLISTER PACK CARTON
- NDC 0004-0800-85
- Tamiflu®
(oseltamivir phosphate) Capsules - 75 mg
- Each capsule contains oseltamivir phosphate equivalent to 75 mg
oseltamivir (free base). - Rx only
- 10 Capsules
- Genentech
- 10175388

PRINCIPAL DISPLAY PANEL – 60 ML BOTTLE CARTON
- NDC 0004-0822-05
- Tamiflu®
(oseltamivir phosphate)
for Oral Suspension - 6 mg/mL
- Each mL contains 6 mg
oseltamivir base after
constitution. - 60 mL (usable volume
after constitution) - Rx only
- Genentech
- 10240835
