Tasigna
Generic name: nilotinib
Drug class: BCR-ABL tyrosine kinase inhibitors
Medically reviewed by A Ras MD.
What is Tasigna?
Tasigna is a prescription medicine used to treat:
- adults and children who have been newly diagnosed with a certain type of leukemia called Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) in chronic phase.
- adults with chronic phase Ph+ CML or accelerated phase Ph+ CML who:
- are no longer benefiting from other treatments, including imatinib (Gleevec), or
- have taken other treatments, including imatinib (Gleevec), and cannot tolerate them.
- children with chronic phase Ph+ CML who:
- are no longer benefiting from treatment with a tyrosine-kinase inhibitor medicine, or
- have taken a tyrosine-kinase inhibitor medicine and cannot tolerate it.
It is not known if Tasigna is safe and effective in children younger than 1 year of age with newly diagnosed, resistant, or intolerant Ph+ CML in chronic phase.
The long-term effects of treating children with Tasigna for a long period of time are not known.
Description
Tasigna contains nilotinib, which belongs to a pharmacologic class of drugs known as kinase inhibitors.
Nilotinib drug substance, in the form of monohydrochloride monohydrate, is a white to slightly yellowish to slightly greenish yellow powder with the molecular formula and weight, respectively, of C28H22F3N7O•HCl • H2O and 584 g/mol (corresponding molecular formula and weight of nilotinib base, anhydrous are C28H22F3N7O and 529 g/mol, respectively). The solubility of nilotinib in aqueous solutions decreases with increasing pH. Nilotinib is not optically active. The pKa1 was determined to be 2.1; pKa2 was estimated to be 5.4.
The chemical name of nilotinib monohydrochloride monohydrate is 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-benzamide, monohydrochloride, monohydrate.
Structure Formula
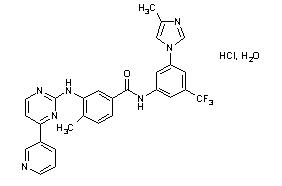
Tasigna (nilotinib) capsules, for oral use, contain 50 mg, 150 mg, or 200 mg nilotinib base, anhydrous (equivalent to 55 mg, 166 mg, and 221 mg nilotinib monohydrochloride monohydrate respectively) with the following inactive ingredients: colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, and poloxamer 188. The capsules contain gelatin, iron oxide (red), iron oxide (yellow), iron oxide (black), and titanium dioxide.
Mechanism of Action
Nilotinib is an inhibitor of the BCR-ABL kinase. Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL protein. In vitro, nilotinib inhibited BCR-ABL mediated proliferation of murine leukemic cell lines and human cell lines derived from patients with Ph+ CML. Under the conditions of the assays, nilotinib was able to overcome imatinib resistance resulting from BCR-ABL kinase mutations, in 32 out of 33 mutations tested. Nilotinib inhibited the autophosphorylation of the following kinases at IC50 values as indicated: BCR-ABL (20 to 60 nM), PDGFR (69 nM), c-KIT (210 nM), CSF-1R (125 to 250 nM), and DDR1 (3.7 nM).
What is the most important information I should know about Tasigna?
Tasigna can cause a possible life-threatening heart problem called QTc prolongation. QTc prolongation causes an irregular heartbeat, which may lead to sudden death.
Your healthcare provider should check the electrical activity of your heart with a test called an electrocardiogram (ECG):
- before starting Tasigna
- 7 days after starting Tasigna
- with any dose changes
- regularly during Tasigna treatment
You may lower your chances for having QTc prolongation with Tasigna if you:
- Take Tasigna on an empty stomach:
- Avoid eating food for at least 2 hours before the dose is taken, and
- Avoid eating food for at least 1 hour after the dose is taken.
- Avoid grapefruit, grapefruit juice, and any supplement containing grapefruit extract during treatment with Tasigna. Food and grapefruit products increase the amount of Tasigna in your body.
- Avoid taking other medicines or supplements with Tasigna that can also cause QTc prolongation.
- Tasigna can interact with many medicines and supplements and increase your chance for serious and life-threatening side effects.
- Do not take any other medicine during treatment with Tasigna unless your healthcare provider tells you it is okay to do so.
- If you cannot swallow Tasigna capsules whole, you may open the Tasigna capsule and sprinkle the contents of each capsule in 1 teaspoon of applesauce (puréed apple). Swallow the mixture right away (within 15 minutes). For more information, see “How should I take Tasigna?”
Call your healthcare provider right away if you feel lightheaded, faint, or have an irregular heartbeat during treatment with Tasigna. These can be symptoms of QTc prolongation.
Who should not take Tasigna?
Do not take if you have:
- low levels of potassium or magnesium in your blood
- long QTc syndrome
What should I tell my healthcare provider before taking Tasigna?
Before taking Tasigna, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems
- have had a stroke or other problems due to decreased blood flow to the brain
- have problems with decreased blood flow to your legs
- have irregular heartbeat
- have QTc prolongation or a family history of it
- have liver problems
- have had pancreatitis
- have low blood levels of potassium or magnesium in your blood
- have a severe problem with lactose (milk sugar) or other sugars. Tasigna capsules contain lactose. Most people who have mild or moderate lactose intolerance can take Tasigna.
- have bleeding problems
- had a surgical procedure involving the removal of the entire stomach (total gastrectomy)
- are pregnant or plan to become pregnant. Tasigna can harm your unborn baby. Tell your healthcare provider right away if you are pregnant, or if you become pregnant during treatment with Tasigna.
In females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with Tasigna.
- Use effective birth control (contraception) during treatment with Tasigna and for at least 14 days after the last dose.
- are breastfeeding or plan to breastfeed. It is not known if Tasigna passes into your breast milk. Do not breastfeed during treatment and for at least 14 days after your last dose of Tasigna.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
If you need to take antacids (medicines to treat heartburn) do not take them at the same time that you take Tasigna. If you take:
- a medicine to block the amount of acid produced in the stomach (H2 blocker): Take these medicines about 10 hours before you take Tasigna, or about 2 hours after you take Tasigna.
- an antacid that contains aluminum hydroxide, magnesium hydroxide, and simethicone to reduce the amount of acid in the stomach: Take these medicines about 2 hours before or about 2 hours after you take Tasigna.
Tasigna can interact with many medicines and supplements and increase your chance for serious and life-threatening side effects. See “What is the most important information I should know about Tasigna?”
How should I take Tasigna?
- Take Tasigna exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking Tasigna unless your healthcare provider tells you.
- Tasigna is a long-term treatment.
- Your healthcare provider will tell you how many Tasigna capsules to take and when to take them.
- If your child takes Tasigna, your healthcare provider will change the dose as your child grows.
- Tasigna must be taken on an empty stomach.
- Avoid eating food for at least 2 hours before the dose is taken, and
- Avoid eating food for at least 1 hour after the dose is taken.
- Swallow Tasigna capsules whole with water. If you cannot swallow Tasigna capsules whole, tell your healthcare provider.
- If you cannot swallow Tasigna capsules whole:
- Open the Tasigna capsules and sprinkle the contents in 1 teaspoon of applesauce (puréed apple).
- Do not use more than 1 teaspoon of applesauce.
- Only use applesauce. Do not sprinkle Tasigna onto other foods.
- Swallow the mixture right away (within 15 minutes).
- Do not drink grapefruit juice, eat grapefruit, or take supplements containing grapefruit extract at any time during treatment. See “What is the most important information I should know about Tasigna?”
- If you miss a dose, just take your next dose at your regular time. Do not take 2 doses at the same time to make up for a missed dose.
- If you take too much Tasigna, call your healthcare provider or go to the nearest hospital emergency room right away. Symptoms may include vomiting and drowsiness.
- During treatment with Tasigna your healthcare provider will do tests to check for side effects and to see how well Tasigna is working for you. The tests will check your:
- heart
- blood cells (white blood cells, red blood cells, and platelets). Your blood cells should be checked every 2 weeks for the first 2 months and then monthly.
- electrolytes (potassium, magnesium)
- pancreas and liver function
- bone marrow samples
Your healthcare provider may change your dose. Your healthcare provider may have you stop Tasigna for some time or lower your dose if you have side effects with it.
- Your healthcare provider will monitor your CML during treatment with Tasigna to see if you are in a remission. After at least 3 years of treatment with Tasigna, your healthcare provider may do certain tests to determine if you continue to be in remission. Based on your test results, your healthcare provider may decide if you may be eligible to try stopping treatment with Tasigna. This is called Treatment Free Remission (TFR).
- Your healthcare provider will carefully monitor your CML during and after you stop taking Tasigna. Based on your test results, your healthcare provider may need to re-start your Tasigna if your CML is no longer in remission.
- It is important that you are followed by your healthcare provider and undergo frequent monitoring to find out if you need to re-start your Tasigna treatment because you are no longer in TFR. Follow your healthcare provider’s instructions about re-starting Tasigna if you are no longer in TFR.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-0951-66
- 120 capsules
- Rx Only
- Tasigna®
(nilotinib) capsules - 50 mg
- Dispense with Medication Guide
- NOVARTIS
PRINCIPAL DISPLAY PANEL
- NDC 0078-0592-87
- Tasigna®
(nilotinib) capsules - 150 mg
per capsule - DISPENSE WITH MEDICATION GUIDE
ATTACHED OR PROVIDED SEPARATELY. - NOVARTIS
PRINCIPAL DISPLAY PANEL
- NDC 0078-0526-87
- Tasigna®
(nilotinib) capsules - 200 mg
per capsule - DISPENSE WITH MEDICATION GUIDE
ATTACHED OR PROVIDED SEPARATELY. - NOVARTIS
What are the possible side effects of Tasigna?
- See “What is the most important information I should know about Tasigna?”
- Low blood cell counts. Low blood cell counts (red blood cells, white blood cells, and platelets) are common with Tasigna, but can also be severe. Your healthcare provider will check your blood counts regularly during treatment with Tasigna. Call your healthcare provider or get medical help right away if you develop any signs or symptoms of low blood counts including:
- fever
- chills or other signs of infection
- unexplained bleeding or bruising
- unexplained weakness
- shortness of breath
- Decreased blood flow to the leg, heart, or brain. People who have recently been diagnosed with Ph+ CML and take Tasigna may develop decreased blood flow to the leg, the heart, or brain.
- Get medical help right away if you suddenly develop any of the following symptoms:
- chest pain or discomfort
- numbness or weakness
- problems walking or speaking
- leg pain
- your leg feels cold
- change in the skin color of your leg
- Pancreas inflammation (pancreatitis). Tell your healthcare provider right away if you develop any symptoms of pancreatitis including sudden stomach area pain with nausea and vomiting.
- Liver problems. Tasigna can increase your risk of liver problems. People who have had liver problems in the past may be at risk for getting liver problems with Tasigna. Call your healthcare provider or get medical help right away if you develop any symptoms of liver problems including:
- stomach area (abdominal) pain
- yellow skin and eyes
- dark-colored urine
- Tumor Lysis Syndrome (TLS). TLS is caused by a fast breakdown of cancer cells. Your healthcare provider may do blood tests to check you for TLS. TLS can cause you to have:
- kidney failure and the need for dialysis treatment
- an abnormal heart beat
- Bleeding problems. Serious bleeding problems and death have happened during treatment with Tasigna. Tell your healthcare provider right away if you develop any signs and symptoms of bleeding during treatment with Tasigna.
- Fluid retention. Your body may hold too much fluid (fluid retention). Symptoms of fluid retention include shortness of breath, rapid weight gain, and swelling.
- Abnormal growth or development in children. Effects on growth and development have happened in children with chronic phase Ph+ CML during treatment with Tasigna. Some children and adolescents may have slower than normal growth during treatment with Tasigna
The most common side effects of Tasigna in adults and children include:
- nausea
- rash
- headache
- tiredness
- itching
- vomiting
- diarrhea
- cough
- constipation
- muscle and joint pain
- runny or stuffy nose, sneezing, sore throat
- fever
- night sweats
Side effects in adult patients attempting treatment free remission:
If you and your healthcare provider decide that you can stop taking Tasigna and try treatment free remission (TFR), you may have more muscle and bone (musculoskeletal) symptoms than before you stopped treatment. Symptoms may include:
- muscle pain
- arm and leg pain
- joint pain
- bone pain
- spine pain
Tell your healthcare provider if you or your child have any side effect that bothers you or does not go away.
These are not all of the possible side effects of Tasigna.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Tasigna?
- Store Tasigna at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away medicine that is out of date or no longer needed.
Keep Tasigna and all medicines out of the reach of children.
What are the ingredients in Tasigna?
Active ingredient: nilotinib
Inactive ingredients: colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate and poloxamer 188.
The capsules contain gelatin, iron oxide (red), iron oxide (yellow), iron oxide (black), and titanium dioxide.
SRC: NLM .

