Temsirolimus
Generic name: temsirolimus
Brand name: Torisel
Dosage form: intravenous solution (25 mg/mL)
Drug class: MTOR inhibitors
Medically reviewed by A Ras MD.
What is temsirolimus?
Temsirolimus is a prescription medicine that is used to treat kidney cancer. Temsirolimus may be given to you for other reasons.
Description
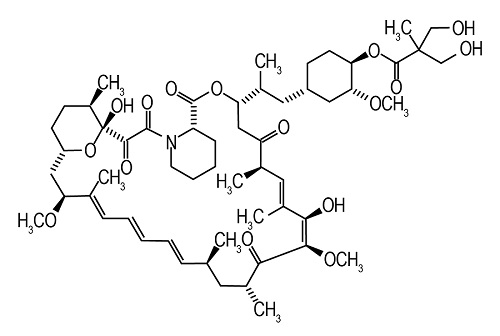
Temsirolimus, an inhibitor of mTOR, is an antineoplastic agent.
Temsirolimus is a white to off-white powder with a molecular formula of C 56H 87NO 16 and a molecular weight of 1030.30. It is non-hygroscopic. Temsirolimus is practically insoluble in water and soluble in alcohol. It has no ionizable functional groups, and its solubility is independent of pH.
The chemical name of temsirolimus is (3 S,6 R,7 E,9 R,10 R,12 R,14 S,15 E,17 E,19 E,21 S,23 S,26 R,27 R,34a S)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1 R)-2-[(1 S,3 R,4 R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3 H-pyrido[2,1- c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4 H,6 H,31 H)-pentone 4′-[2,2-bis(hydroxymethyl)propionate]; or Rapamycin, 42-[3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate].
Temsirolimus injection, 25 mg/mL, is a clear, colorless to light yellow, non-aqeous, ethanolic, sterile solution. Temsirolimus injection requires two dilutions prior to intravenous infusion. Temsirolimus injection should be diluted only with the supplied Diluent for Temsirolimus injection.
Diluent for Temsirolimus injection is a sterile, non-aqueous solution that is supplied with Temsirolimus injection, as a kit.
- Temsirolimus injection, 25 mg/mL:
- Active ingredient: temsirolimus (25 mg/mL)
Inactive ingredients: dehydrated alcohol (39.5% w/v), butylated hydroxyanisole (0.0003% w/v), butylated hydroxytoluene (0.001% w/v), propylene glycol (50.3% w/v), and anhydrous citric acid (0.0025% w/v).
Diluent for Temsirolimus injection
Inactive ingredients: polysorbate 80 (40.0% w/v), polyethylene glycol 400 (42.8% w/v) and dehydrated alcohol (19.9% w/v).
Temsirolimus injection and Diluent for Temsirolimus injection are filled in clear glass vials with rubber stoppers.
Mechanism of Action
Temsirolimus is an inhibitor of mTOR (mammalian target of rapamycin). Temsirolimus binds to an intracellular protein (FKBP-12), and the protein-drug complex inhibits the activity of mTOR that controls cell division. Inhibition of mTOR activity resulted in a G1 growth arrest in treated tumor cells. When mTOR was inhibited, its ability to phosphorylate p70S6k and S6 ribosomal protein, which are downstream of mTOR in the PI3 kinase/AKT pathway was blocked. In in vitro studies using renal cell carcinoma cell lines, temsirolimus inhibited the activity of mTOR and resulted in reduced levels of the hypoxia-inducible factors HIF-1 and HIF-2 alpha, and the vascular endothelial growth factor.
Before taking temsirolimus, tell your doctor:
- If you are allergic to this medicine (temsirolimus); any part of this medicine (temsirolimus); or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have a high bilirubin level or liver problems.
- If you are taking sunitinib.
- If you take any other drugs (prescription or OTC, natural products, vitamins). There are many drugs that interact with this medicine (temsirolimus), like certain drugs that are used for HIV, infections, or seizures.
- If you are taking St. John’s wort. Do not take St. John’s wort with this medicine (temsirolimus). This medicine may not work as well.
- If you are breast-feeding. Do not breast-feed while you take this medicine (temsirolimus) and for 3 weeks after your last dose.
This is not a list of all drugs or health problems that interact with this medicine (temsirolimus).
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this medicine (temsirolimus) with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take temsirolimus?
- Tell all of your health care providers that you take this medicine (temsirolimus). This includes your doctors, nurses, pharmacists, and dentists.
- Avoid grapefruit and grapefruit juice.
- You may have more of a chance of getting an infection. Wash hands often. Stay away from people with infections, colds, or flu. Some infections have been very bad and even deadly.
- Call your doctor right away if you have any signs of infection like fever, chills, flu-like signs, very bad sore throat, ear or sinus pain, cough, more sputum or change in color of sputum, pain with passing urine, mouth sores, or a wound that will not heal.
- You may bleed more easily. Be careful and avoid injury. Use a soft toothbrush and an electric razor.
- Talk with your doctor before getting any vaccines. Use of some vaccines with this medicine (temsirolimus) may either raise the chance of an infection or make the vaccine not work as well.
- If you have high blood sugar (diabetes), talk with your doctor. This medicine may raise blood sugar.
- Check your blood sugar as you have been told by your doctor.
- This medicine may cause high cholesterol and triglyceride levels. Talk with the doctor.
- People taking this medicine (temsirolimus) who have brain or nervous system tumors may have a raised chance of bleeding in the brain. The chance may also be raised in people who are taking blood thinners with this medicine (temsirolimus). Sometimes, this may be deadly. Talk with your doctor.
- Very bad and sometimes deadly holes in the GI (gastrointestinal) tract have happened with this medicine (temsirolimus). Talk with the doctor.
- Very bad and sometimes deadly infusion reactions have happened with this medicine (temsirolimus). Call your doctor right away if you have chest pain, flushing, shortness of breath, very bad dizziness, or you pass out.
- Blood clots have happened with this medicine (temsirolimus). Sometimes, these blood clots have been deadly. Talk with the doctor.
- Call your doctor right away if you have signs of a blood clot like chest pain or pressure; coughing up blood; shortness of breath; swelling, warmth, numbness, change of color, or pain in a leg or arm; or trouble speaking or swallowing.
- If you are 65 or older, use this medicine (temsirolimus) with care. You could have more side effects.
- This medicine may affect fertility. Fertility problems may lead to not being able to get pregnant or father a child.
- If you are a man and have sex with a female who could get pregnant, protect her from pregnancy during treatment and for 3 months after your last dose.
- If you are a man and your sex partner gets pregnant while you take this medicine (temsirolimus) or within 3 months after your last dose, call your doctor right away.
- This medicine may cause harm to the unborn baby if you take it while you are pregnant.
- Use birth control to prevent pregnancy while taking this medicine (temsirolimus) and for 3 months after the last dose.
- If you get pregnant while taking this medicine (temsirolimus) or within 3 months after your last dose, call your doctor right away.
How is temsirolimus best taken?
Use this medicine (temsirolimus) as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as an infusion into a vein over a period of time.
- Other drugs may be given before this medicine (temsirolimus) to help avoid side effects.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Have your urine checked as you have been told by your doctor.
- This medicine may affect how wounds heal. If you have surgery, you may need to stop this medicine (temsirolimus) before surgery. Start taking it again after surgery as you are told by your doctor.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of temsirolimus that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of high blood sugar like confusion, feeling sleepy, more thirst, more hungry, passing urine more often, flushing, fast breathing, or breath that smells like fruit.
- Signs of too much acid in the blood (acidosis) like confusion; fast breathing; fast heartbeat; a heartbeat that does not feel normal; very bad stomach pain, upset stomach, or throwing up; feeling very sleepy; shortness of breath; or feeling very tired or weak.
- Signs of high blood pressure like very bad headache or dizziness, passing out, or change in eyesight.
- Signs of electrolyte problems like mood changes, confusion, muscle pain or weakness, a heartbeat that does not feel normal, seizures, not hungry, or very bad upset stomach or throwing up.
- Feeling very tired or weak.
- Any unexplained bruising or bleeding.
- Black, tarry, or bloody stools.
- Stomach pain.
- Diarrhea.
- Swelling.
- Some people have had lung problems with this medicine (temsirolimus). Sometimes, this has been deadly. Call your doctor right away if you have signs of lung problems like shortness of breath or other trouble breathing, cough that is new or worse, or fever.
- Very bad and sometimes deadly kidney problems have happened with this medicine (temsirolimus). Call your doctor right away if you are unable to pass urine or if you have blood in the urine or a change in the amount of urine passed.
What are some other side effects of temsirolimus?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Pimples (acne).
- Upset stomach or throwing up.
- Mouth irritation or mouth sores.
- Constipation.
- Headache.
- Back pain.
- Muscle or joint pain.
- Not able to sleep.
- Dry skin.
- Change in nails.
- Nosebleed.
- Runny nose.
- Throat irritation.
- Not hungry.
- Change in taste.
- Weight loss.
- Feeling tired or weak.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out temsirolimus?
- If you need to store this medicine (temsirolimus) at home, talk with your doctor, nurse, or pharmacist about how to store it.
Label
PRINCIPAL DISPLAY PANEL – 25 mg/mL Vial Label
- NDC 16729- 221-30
- Temsirolimus Injection
- 25 mg/mL*
- *each vial contains 0.2 mL overfill
- 10 mg/mL after Initial dilution
- MUST BE DILUTED
- Single Dose – Refrigerate
- Rx Only
PRINCIPAL DISPLAY PANEL – 2.2 mL Vial Label
- NDC 16729- 222-30
- Diluent for Temsirolimus Injection
- 2.2 mL
- Rx Only
- Not for direct administration
- Only for dilution of Temsirolimus injection vial
- Single Dose – Refrigerate

PRINCIPAL DISPLAY PANEL – Kit Carton
- NDC 16729- 223-61
- Temsirolimus Injection
- 25 mg/mL per vial*
- *each single dose vial contains 0.2 mL overfill
- CONCENTRATED – Requires two dilutions before administration
- 10 mg/mL after Initial dilution
- For intravenous infusion only
- Each carton contains:
- 1 Single Dose vial Temsirolimus injection 25 mg/mL*
- 1 Single Dose vial DILUENT for Temsirolimus injections
- Refrigerate
- Rx Only
SRC: NLM .