Tikosyn
Generic name: dofetilide
Drug class: Group III antiarrhythmics
Medically reviewed by A Ras MD.
What is Tikosyn?
Tikosyn is a prescription medicine that is used to treat an irregular heartbeat (atrial fibrillation or atrial flutter).
It is not known if Tikosyn is safe and effective in children under 18 years of age.
Description
TIKOSYN® (dofetilide) is an antiarrhythmic drug with Class III (cardiac action potential duration prolonging) properties. Its empirical formula is C19H27N3O5S2 and it has a molecular weight of 441.6. The structural formula is
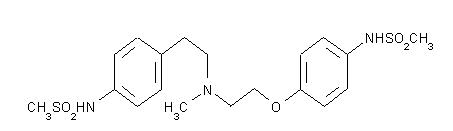
The chemical name for dofetilide is:
N-[4-[2-[methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]-methanesulfonamide.
Dofetilide is a white to off-white powder. It is very slightly soluble in water and propan-2-ol and is soluble in 0.1M aqueous sodium hydroxide, acetone, and aqueous 0.1M hydrochloric acid.
TIKOSYN capsules contain the following inactive ingredients: microcrystalline cellulose, corn starch, colloidal silicon dioxide and magnesium stearate. TIKOSYN is supplied for oral administration in three dosage strengths: 125 mcg (0.125 mg) orange and white capsules, 250 mcg (0.25 mg) peach capsules, and 500 mcg (0.5 mg) peach and white capsules.
Mechanism of Action
Dofetilide shows Vaughan Williams Class III antiarrhythmic activity. The mechanism of action is blockade of the cardiac ion channel carrying the rapid component of the delayed rectifier potassium current, IKr. At concentrations covering several orders of magnitude, dofetilide blocks only IKr with no relevant block of the other repolarizing potassium currents (e.g., IKs, IK1). At clinically relevant concentrations, dofetilide has no effect on sodium channels (associated with Class I effect), adrenergic alpha-receptors, or adrenergic beta-receptors.
What is the most important information I should know about Tikosyn?
Tikosyn can cause serious side effects, including a type of abnormal heartbeat called Torsade de Pointes, which can lead to death.
To establish the right dose of Tikosyn, treatment with Tikosyn must be started in a hospital where your heart rate and kidney function will be checked for the first 3 days of treatment. It is important that when you go home, you take the exact dose of Tikosyn that your doctor prescribed for you.
While you take Tikosyn, always watch for signs of abnormal heartbeat.
Call your doctor and go to the hospital right away if you:
- feel faint
- become dizzy, or
- have a fast heartbeat
Who should not take Tikosyn?
Do not take Tikosyn if you:
- have an irregular heartbeat called long QT syndrome
- have kidney problems or are on kidney dialysis
- take any of these medicines:
- cimetidine (Tagamet, Tagamet HB)
- verapamil (Calan, Calan SR, Covera-HS, Isoptin, Isoptin SR, Verelan, Verelan PM, Tarka)
- ketoconazole (Nizoral, Xolegel, Extina)
- trimethoprim alone (Proloprim, Trimpex) or the combination of trimethoprim and sulfamethoxazole (Bactrim, Septra Sulfatrim)
- prochlorperazine (Compazine, Compo)
- megestrol (Megace)
- dolutegravir (Tivicay)
- hydrochlorothiazide alone or in combination with other medicines (such as Esidrix, Ezide, Hydrodiuril, Hydro-Par, Microzide, or Oretic)
Ask your doctor if you are not sure if any of your medicines are the kind listed above.
- are allergic to dofetilide in Tikosyn. See below for a complete list of ingredients in Tikosyn.
What should I tell my healthcare provider before taking Tikosyn?
Before taking Tikosyn, tell your doctor about all of your medical conditions including if you:
- have heart problems
- have kidney or liver problems
- are pregnant or plan to become pregnant. It is not known if Tikosyn will harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if Tikosyn passes into your breast milk. You and your doctor should decide if you will take Tikosyn or breast-feed. You should not do both.
Especially tell your doctor if you take medicines to treat:
- heart problems
- high blood pressure
- depression or other mental problems
- asthma
- allergies, or hay fever
- skin problems
- infections
Ask your doctor if you are not sure about the medicines you take. Tell your doctor about all prescription and non-prescription medicines, vitamins, dietary supplements, and any natural or herbal remedies. Tikosyn and other medicines may affect each other, causing serious side effects. If you take Tikosyn with certain medicines, you will be more likely to have a different type of abnormal heartbeat. See “Who should not take Tikosyn?”
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take Tikosyn?
- Take Tikosyn exactly as your doctor tells you.
- Do not change your Tikosyn dose unless your doctor tells you to.
- Your doctor will do tests before you start and while you take Tikosyn.
- Do not stop taking Tikosyn until your doctor tells you to stop. If you miss a dose, just take the next dose at your regular time. Do not take 2 doses of Tikosyn at the same time.
- Tikosyn can be taken with or without food.
- If you take too much Tikosyn, call your doctor or go to the nearest hospital emergency room right away. Take your Tikosyn capsules with you to show to the doctor.
What are the possible side effects of Tikosyn?
Tikosyn can cause serious side effects, including a type of abnormal heartbeat called Torsade de Pointes, which can lead to death. See “What is the most important information I should know about Tikosyn?”
The most common side effects of Tikosyn include:
- headache
- chest pain
- dizziness
Call your doctor right away if you have signs of electrolyte imbalance:
- severe diarrhea
- unusual sweating
- vomiting
- not hungry (loss of appetite)
- increased thirst (drinking more than normal)
Tell your doctor if you have any side effects that bother you or do not go away.
These are not all the possible side effects of Tikosyn. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tikosyn
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Tikosyn for a condition for which it was not prescribed. Do not give Tikosyn to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about Tikosyn. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Tikosyn that is written for health professionals.
For more about Tikosyn, go to www.Tikosyn.com or call 1-877-Tikosyn (1-877-845-6796).
How should I store Tikosyn?
- Store Tikosyn between 59° to 86°F (15° to 30°C).
- Keep Tikosyn away from moisture and humidity.
- Keep Tikosyn in a tightly closed container.
- Keep Tikosyn and all medicines out of the reach of children.
What are the ingredients in Tikosyn?
Active ingredient: dofetilide
Inactive ingredients:
- Capsule fill: microcrystalline cellulose, corn starch, colloidal silicon dioxide, and magnesium stearate
- Capsule shell: gelatin, titanium dioxide, and FD&C Yellow 6
- Imprinting ink: iron oxide black, shellac, n-butyl alcohol, isopropyl alcohol, propylene glycol, and ammonium hydroxide
Label
PRINCIPAL DISPLAY PANEL – 0.125 MG CAPSULE BLISTER PACK CARTON
- ALWAYS DISPENSE WITH MEDICATION GUIDE
- Pfizer
NDC 0069-5800-43 - Tikosyn®
- (dofetilide) capsules
- 125 mcg (0.125 mg)
- For in-institution use only
- 40 Capsules
- Rx only
UNIT DOSE


PRINCIPAL DISPLAY PANEL – 0.25 MG CAPSULE BOTTLE LABEL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
- Pfizer
NDC 0069-5810-60 - Tikosyn®
- (dofetilide) Capsules
- 250 mcg (0.25 mg)
- 60 Capsules
Rx only


PRINCIPAL DISPLAY PANEL – 0.5 MG CAPSULE BOTTLE LABEL
- ALWAYS DISPENSE WITH MEDICATION GUIDE
- Pfizer
NDC 0069-5820-60 - Tikosyn®
- (dofetilide) Capsules
- 500 mcg (0.5 mg)
- 60 Capsules
Rx only


SRC: NLM .