Umeclidinium
Generic name: umeclidinium
Brand name: Incruse Ellipta
Dosage form: inhalation powder (62.5 mcg (0.0625 mg)/inh)
Drug class: Anticholinergic bronchodilators
Medically reviewed by A Ras MD.
What is umeclidinium?
Umeclidinium is used to treat COPD (chronic obstructive pulmonary disease). This medicine is not to be used to treat intense flare-ups of shortness of breath. Use a rescue inhaler.
Description
ANORO ELLIPTA is an inhalation powder drug product for delivery of a combination of umeclidinium (an anticholinergic) and vilanterol (a LABA) to patients by oral inhalation.
Umeclidinium bromide has the chemical name 1-[2-(benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-azoniabicyclo[2.2.2]octane bromide and the following chemical structure:
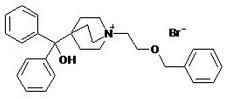
Umeclidinium bromide is a white powder with a molecular weight of 508.5, and the empirical formula is C29H34NO2•Br (as a quaternary ammonium bromide compound). It is slightly soluble in water.
Vilanterol trifenatate has the chemical name triphenylacetic acid-4-{(1R)-2-[(6-{2-[(2,6-dicholorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol (1:1) and the following chemical structure:
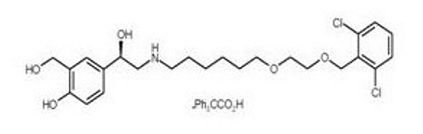
Vilanterol trifenatate is a white powder with a molecular weight of 774.8, and the empirical formula is C24H33Cl2NO5•C20H16O2. It is practically insoluble in water.
ANORO ELLIPTA is a light grey and red plastic inhaler containing 2 foil blister strips. Each blister on one strip contains a white powder mix of micronized umeclidinium bromide (74.2 mcg equivalent to 62.5 mcg of umeclidinium), magnesium stearate (75 mcg), and lactose monohydrate (to 12.5 mg), and each blister on the other strip contains a white powder mix of micronized vilanterol trifenatate (40 mcg equivalent to 25 mcg of vilanterol), magnesium stearate (125 mcg), and lactose monohydrate (to 12.5 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within both blisters is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, ANORO ELLIPTA delivers 55 mcg of umeclidinium and 22 mcg of vilanterol per dose when tested at a flow rate of 60 L/min for 4 seconds.
In adult subjects with obstructive lung disease and severely compromised lung function (COPD with FEV1/FVC <70% and FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 66.5 L/min (range: 43.5 to 81.0 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
Mechanism of Action
Umeclidinium
Umeclidinium is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptors at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine- and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of umeclidinium is predominantly a site-specific effect.
Before taking umeclidinium, tell your doctor:
- If you are allergic to umeclidinium; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have a milk allergy.
- If you take other drugs called anticholinergics, like ipratropium or oxybutynin. Ask your doctor if you are not sure if any of your drugs are anticholinergic.
This is not a list of all drugs or health problems that interact with umeclidinium.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take umeclidinium with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take umeclidinium?
- Tell all of your health care providers that you take umeclidinium. This includes your doctors, nurses, pharmacists, and dentists.
- Call your doctor right away if your breathing problems get worse, if your rescue inhaler does not work as well, or if you need to use your rescue inhaler more often.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is umeclidinium best taken?
Use umeclidinium as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- For breathing in only.
- Take umeclidinium at the same time of day.
- Keep using umeclidinium as you have been told by your doctor or other health care provider, even if you feel well.
- Do not take out the inhaler from the foil tray until right before first use.
- If you are using more than 1 inhaled drug, ask the doctor which drug to use first.
- Do not breathe out into the inhaler. Close the inhaler after you use your dose.
- Do not take the device apart or wash it. Do not use it with a spacer. Do not breathe out into the device.
- Clean mouthpiece by wiping with a dry tissue or cloth. Do not wash or put in water.
What do I do if I miss a dose?
- Use a missed dose as soon as you think about it.
- If it is close to the time for your next dose, skip the missed dose and go back to your normal time.
- Do not take more than 1 dose of umeclidinium in 24 hours.
What are the side effects of umeclidinium that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Bruising or dark areas of skin.
- Chest pain or pressure.
- Fast or abnormal heartbeat.
- Shakiness.
- Change in eyesight, eye pain, or very bad eye irritation.
- Seeing halos or bright colors around lights.
- Eye redness.
- Trouble passing urine, pain when passing urine, passing urine in a weak stream or drips, or passing urine more often.
- This medicine can cause very bad breathing problems right after you take a dose. Sometimes, this may be life-threatening. If you have trouble breathing, breathing that is worse, wheezing, or coughing after using umeclidinium, use a rescue inhaler and get medical help right away.
What are some other side effects of umeclidinium?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Nose or throat irritation.
- Signs of a common cold.
- Cough.
- Muscle or joint pain.
- Stomach pain.
- Tooth pain.
- Change in taste.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out umeclidinium?
- Store at room temperature in a dry place. Do not store in a bathroom.
- Protect from heat and light.
- After opening, throw away any part not used after 6 weeks or when the indicator reads zero, whichever comes first.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
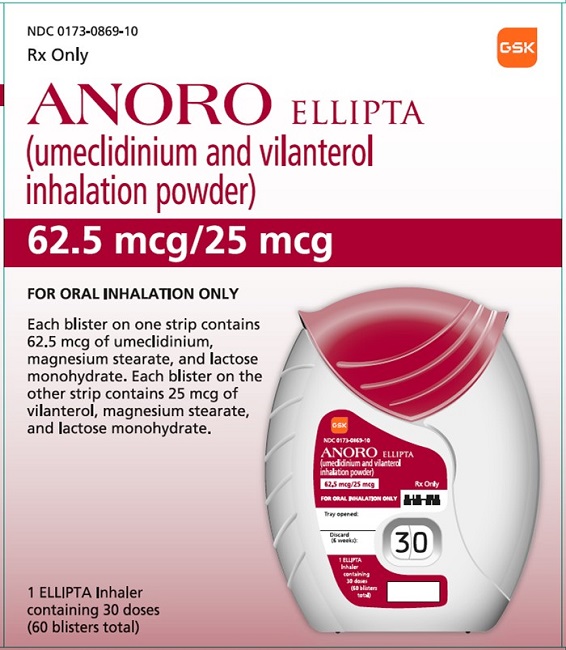
PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- NDC 0173-0869-10
- ANORO ELLIPTA
- (umeclidinium and vilanterol inhalation powder)
- Rx Only
- 62.5 mcg/25 mcg
- FOR ORAL INHALATION ONLY
- Each blister on one strip contains 62.5 mcg of umeclidinium, magnesium stearate, and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
- 1 ELLIPTA Inhaler containing 30 doses (60 blisters total)
- ©2020 GSK group of companies or its licensor.