Valtoco
Generic name: diazepam nasal
Drug class: Benzodiazepine anticonvulsants
Medically reviewed by A Ras MD.
What is Valtoco?
Valtoco is a prescription medicine used for short-term treatment of seizure clusters (also known as “acute repetitive seizures”) in people 6 years of age and older.
Valtoco is a federal controlled substance (C-IV) because it can be abused or lead to dependence. Keep Valtoco in a safe place to prevent misuse and abuse. Selling or giving away Valtoco may harm others and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription drugs, or street drugs.
It is not known if Valtoco is safe and effective in children under 6 years of age.
Description
Diazepam, the active ingredient of VALTOCO nasal spray, is a benzodiazepine anticonvulsant with the chemical name 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. The structural formula is as follows:
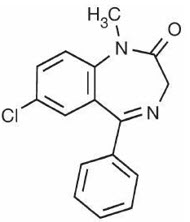
The inactive ingredients in VALTOCO nasal spray include benzyl alcohol (10.5 mg per 0.1 mL), dehydrated alcohol, n-dodecyl beta-D-maltoside, and vitamin E. VALTOCO nasal spray is a clear pale amber liquid.
Mechanism of Action
The exact mechanism of action for diazepam is not fully understood, but it is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
What is the most important information I should know about Valtoco?
- Valtoco is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.
- Valtoco can make you sleepy or dizzy and can slow your thinking and motor skills. Do not drive, operate heavy machinery, or do other dangerous activities until you know how Valtoco affects you.
- Like other antiepileptic drugs, Valtoco may cause suicidal thoughts or actions in a small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- feeling agitated or restless
- acting aggressive, being angry, or violent
- attempts to commit suicide
- panic attacks
- acting on dangerous impulses
- trouble sleeping (insomnia)
- an extreme increase in activity and talking (mania)
- new or worse anxiety
- new or worse irritability
- other unusual changes in behavior or mood
- new or worse depression
How can I watch for early symptoms of suicidal thoughts or actions?
- Pay attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
Who should not use Valtoco?
Do not use Valtoco if you:
- are allergic to diazepam or any of the ingredients in Valtoco. See the end of this Medication Guide for a complete list of ingredients in Valtoco.
- have an eye problem called acute narrow angle glaucoma.
What should I tell my healthcare provider before using Valtoco?
Before using Valtoco, tell your healthcare provider about all of your medical conditions, including if you:
- have asthma, emphysema, bronchitis, chronic obstructive pulmonary disease, or other breathing problems.
- have a history of alcohol or drug abuse.
- have a history of depression, mood problems or suicidal thoughts or behaviors.
- have liver or kidney problems.
- are pregnant or plan to become pregnant. Valtoco may harm your unborn baby.
- Babies born to mothers receiving benzodiazepine medicines (including Valtoco) late in pregnancy may be at risk of having breathing problems, feeding problems, dangerously low body temperature, and withdrawal symptoms
- If you become pregnant while using Valtoco, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. You can register by calling 1-888-233-2334. For more information about the registry, go to http://www.aedpregnancyregistry.org. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding or plan to breastfeed. Valtoco passes into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you use Valtoco.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using Valtoco with certain other medicines can cause side effects or affect how well Valtoco or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I use Valtoco?
- Read the Instructions for use that comes with this Medication Guide for detailed information about the right way to use Valtoco.
- Use Valtoco exactly as prescribed by the healthcare provider.
- Your healthcare provider will tell you:
- what seizure clusters are
- exactly how much Valtoco to give
- when to give Valtoco
- how to give Valtoco
- what to do after you give Valtoco if the seizures do not stop or there is a change in breathing, behavior, or condition that worries you
- You should carry Valtoco with you in case you need it to control your seizure clusters.
- Family members, care providers, and other people who may have to give Valtoco should know where you keep your Valtoco and how to give Valtoco before a seizure cluster happens.
- Valtoco is given in the nose (nasal) only.
- Valtoco comes ready to use.
- Each Valtoco only sprays 1 time and cannot be reused. Do not test or prime the nasal spray before use.
- Each dose of Valtoco is provided in an individual pack. Use all of the medicine in 1 pack for a complete dose.
What should I do after I give Valtoco?
- Stay with the person after you give Valtoco and watch them closely.
- Keep or move the person onto their side.
- Make a note of the time Valtoco was given.
- Call for emergency help if any of the following happen:
- seizure behavior is different than other seizures the person has had
- you are alarmed by how often the seizures happen, by how severe the seizure is, by how long the seizure lasts, or by the color or breathing of the person.
- Throw away (discard) the used Valtoco.
If needed, a second dose may be given at least 4 hours after the first dose, using a new pack of Valtoco. Do not give more than 2 doses of Valtoco to treat a seizure cluster.
A second dose should not be given if there is concern about the person’s breathing, they need help with their breathing, or have extreme drowsiness.
Do not use Valtoco for more than 1 seizure cluster episode every 5 days. Do not use Valtoco for more than 5 seizure cluster episodes in 1 month.
What should I avoid while using Valtoco?
- Do not drink alcohol or take opioid medicines or other medicines that make you sleep or dizzy while taking Valtoco until you talk to your healthcare provider. When taken with alcohol or medicines that can cause sleepiness or dizziness Valtoco may make your sleepiness or dizziness worse.
What are the possible side effects of Valtoco?
Valtoco may cause serious side effects, including:
- See “What is the most important information I should know about Valtoco?”
- Increase in eye pressure in people with open-angle glaucoma. See “Who should not use Valtoco?”
The most common side effects of Valtoco include:
- feeling sleepy or drowsy
- headache
- rash
- nose discomfort
These are not all of the possible side effects of Valtoco. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Valtoco
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Valtoco for a condition for which it was not prescribed. Do not give Valtoco to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Valtoco that is written for health professionals.
How should I store Valtoco?
- Store Valtoco at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze Valtoco.
- Keep Valtoco in its box until ready to use. Protect it from light.
- Keep Valtoco and all medicines out of the reach of children.
What are the ingredients in Valtoco?
Active ingredient: diazepam
Inactive ingredients: benzyl alcohol, dehydrated alcohol, n-dodecyl beta-D-maltoside, and vitamin E.
For more information, go to www.valtoco.com or call 1-866-696-3873.
Instructions for use for Valtoco
For 5 mg and 10 mg Doses
(diazepam nasal spray) CIV
Important: For Nasal Use Only.
Check the expiration date before use.
Do not remove Valtoco until ready to use. Do not test Valtoco.
Keep out of reach of children.
Inspect Valtoco for damage. If damaged, you may not receive the full dose.
You and your family members, caregivers, and others who may need to administer Valtoco should read this Instructions for Use that comes with Valtoco before using it. Talk to your healthcare provider if you, your caregiver, or others who may need to administer Valtoco have any questions about the use of Valtoco.
Safely secure the person
If the person appears to be having a seizure, gently help them to the floor and lay them on their side in a place where they cannot fall.
The person can be on either their side or back to receive Valtoco.
Move objects and furniture away from the person to avoid injury.
| Give Valtoco 5 mg dose or 10 mg dose. 1 dose equals 1 nasal spray device.
Device sprays one time only. Important: Do not test or prime Valtoco. |
 |
Step 1: Remove 1 Valtoco blister pack from the box.
Each blister pack contains 1 nasal spray device. 1 device contains 1 dose.
 |
 |
| Peel back the tab with the arrow on the corner of the pack. | Remove Valtoco from the pack. |
Step 2: Hold Valtoco with your thumb on the bottom of the plunger and your first and middle fingers on either side of the nozzle.

Step 3: Gently insert the tip of the nozzle into 1 nostril until your fingers, on either side of the nozzle, are against the bottom of the person’s nose.

Step 4: Press the bottom of the plunger firmly with your thumb to give Valtoco.

Step 5: Remove Valtoco from the nose after giving the dose.
Each individual Valtoco contains 1 single spray.
Throw it away (discard) after use.
After giving Valtoco, evaluate and support
Keep or move the person onto their side, facing you, so that you can watch them closely.
Loosen any tight clothing and provide a safe area where the person can rest.

Call for emergency help if any of the following happen:
- Seizure clusters are different from that of other seizures the person has had
- You are alarmed by how often the seizures happen, by how severe the seizure is, by how long the seizure lasts, or by the color or breathing of the person
Make a note of the time Valtoco was given and continue to watch the person closely.
Time of first Valtoco dose:____________________ Time of second Valtoco dose (if given):____________________
The healthcare provider may prescribe another dose of Valtoco to be given at least 4 hours after the first dose. If a second dose is needed, repeat Steps 1 through 5 with a new blister pack of Valtoco. If the person is not having a seizure when the second dose of Valtoco is given, it may be given to the person when they are lying down, standing, or sitting.
Label
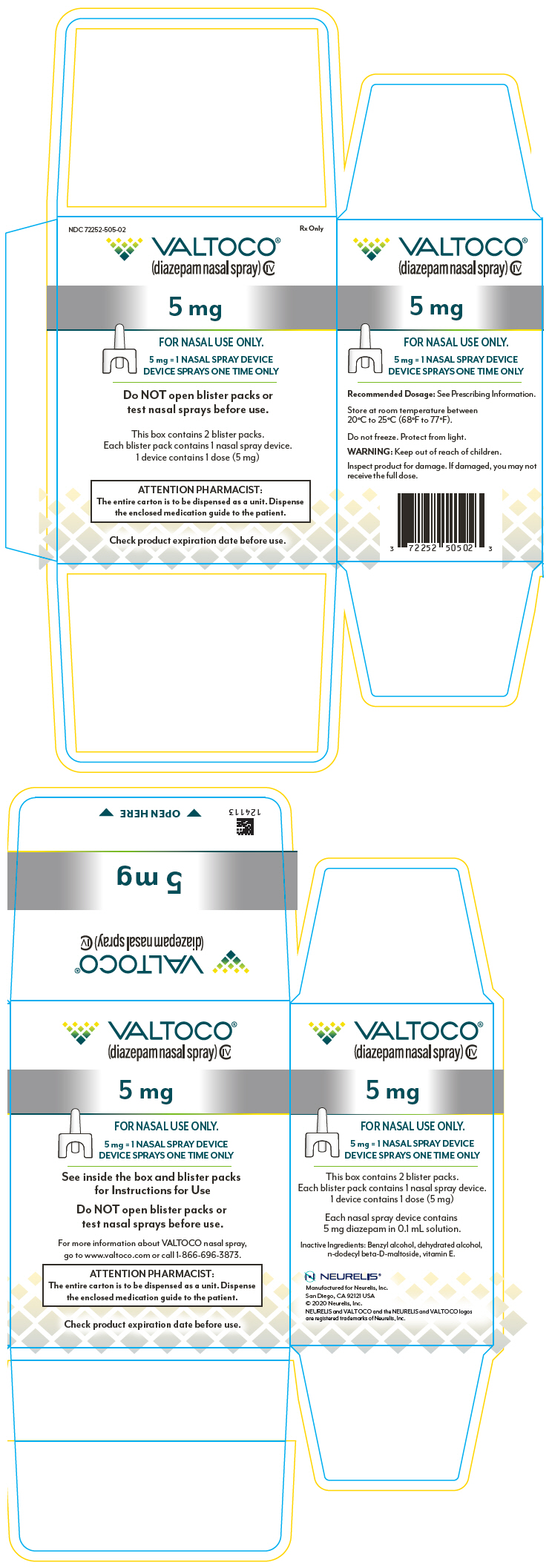
PRINCIPAL DISPLAY PANEL – 5 MG DEVICE BLISTER PACK CARTON
- NDC 72252-505-02
Rx Only - VALTOCO®
(diazepam nasal spray) CIV - 5 mg
- FOR NASAL USE ONLY.
- 5 mg = 1 NASAL SPRAY DEVICE
DEVICE SPRAYS ONE TIME ONLY - Do NOT open blister packs or
test nasal sprays before use. - This box contains 2 blister packs.
Each blister pack contains 1 nasal spray device.
1 device contains 1 dose (5 mg) - ATTENTION PHARMACIST:
The entire carton is to be dispensed as a unit. Dispense
the enclosed medication guide to the patient. - Check product expiration date before use.
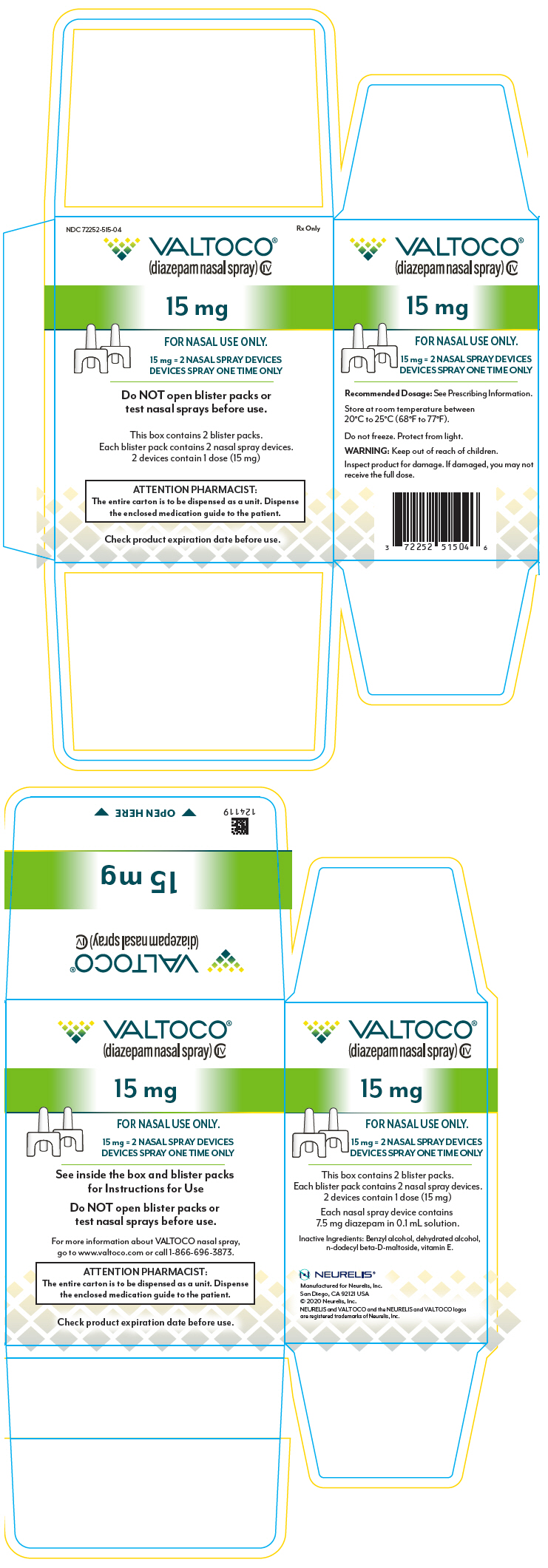
PRINCIPAL DISPLAY PANEL – 15 MG DEVICE BLISTER PACK CARTON
- NDC 72252-515-04
Rx Only - VALTOCO®
(diazepam nasal spray) CIV - 15 mg
- FOR NASAL USE ONLY.
- 15 mg = 2 NASAL SPRAY DEVICES
DEVICES SPRAY ONE TIME ONLY - Do NOT open blister packs or
test nasal sprays before use. - This box contains 2 blister packs.
Each blister pack contains 2 nasal spray devices.
2 devices contains 1 dose (15 mg) - ATTENTION PHARMACIST:
The entire carton is to be dispensed as a unit. Dispense
the enclosed medication guide to the patient. - Check product expiration date before use.
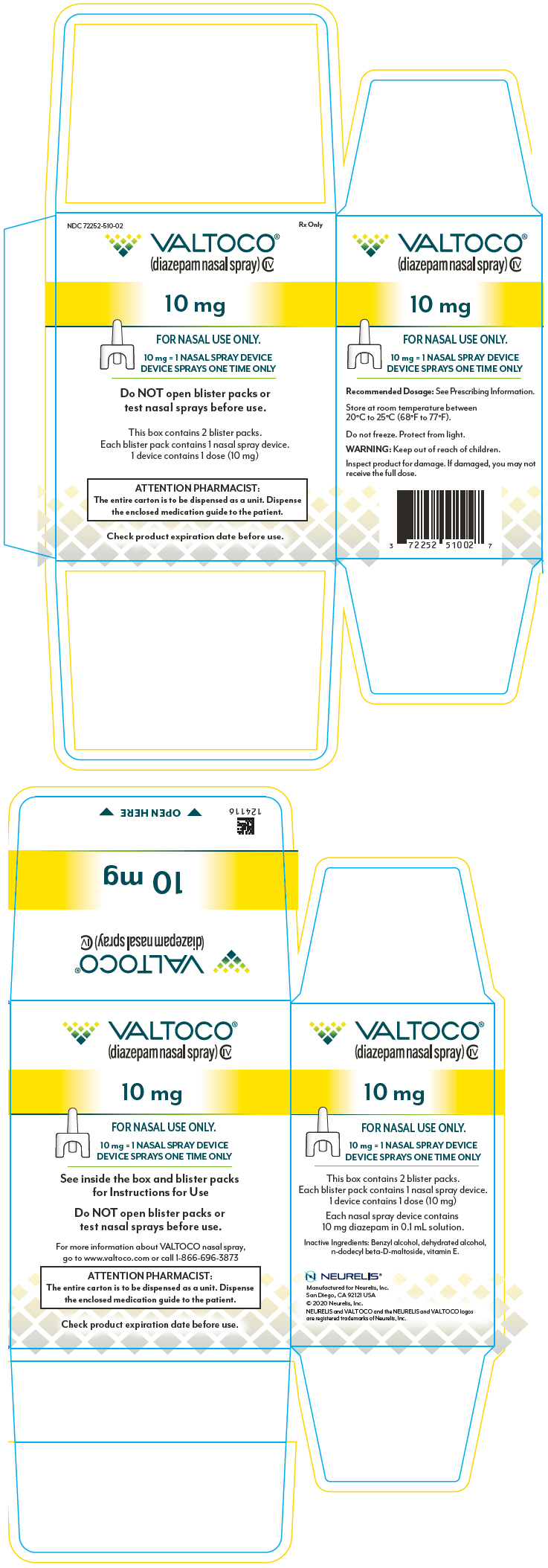
PRINCIPAL DISPLAY PANEL – 10 MG DEVICE BLISTER PACK CARTON
- NDC 72252-510-02
Rx Only - VALTOCO®
(diazepam nasal spray) CIV - 10 mg
- FOR NASAL USE ONLY.
- 10 mg = 1 NASAL SPRAY DEVICE
DEVICE SPRAYS ONE TIME ONLY - Do NOT open blister packs or
test nasal sprays before use. - This box contains 2 blister packs.
Each blister pack contains 1 nasal spray device.
1 device contains 1 dose (10 mg) - ATTENTION PHARMACIST:
The entire carton is to be dispensed as a unit. Dispense
the enclosed medication guide to the patient. - Check product expiration date before use.
SRC: NLM .