Victoza
Generic name: liraglutide
Brand names: Saxenda, Victoza
Drug class: Incretin mimetics
Medically reviewed by A Ras MD.
What is Victoza?
Victoza is an injectable prescription medicine used along with diet and exercise to lower blood sugar (glucose) in adults and children who are 10 years of age and older with type 2 diabetes mellitus.
It is also used to reduce the risk of major cardiovascular events such as heart attack, stroke or death in adults with type 2 diabetes mellitus with known heart disease.
Victoza is not for use in people with type 1 diabetes. It should not be used with other medicines that contain liraglutide. It is not known if Victoza is safe and effective to lower blood sugar (glucose) in children under 10 years of age.
Description
VICTOZA contains liraglutide, an analog of human GLP-1 and acts as a GLP-1 receptor agonist. The peptide precursor of liraglutide, produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae, has been engineered to be 97% homologous to native human GLP-1 by substituting arginine for lysine at position 34. Liraglutide is made by attaching a C-16 fatty acid (palmitic acid) with a glutamic acid spacer on the remaining lysine residue at position 26 of the peptide precursor. The molecular formula of liraglutide is C172H265N43O51 and the molecular weight is 3751.2 Daltons. The structural formula (Figure 1) is:
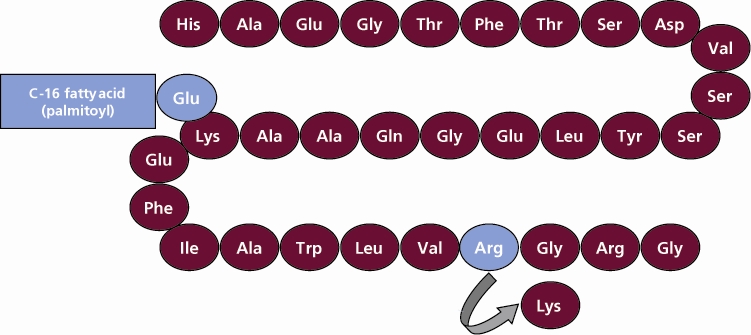
Figure 1 Structural Formula of liraglutide
VICTOZA injection is a sterile, aqueous, clear, colorless or almost colorless solution for subcutaneous use. Each 1 mL of VICTOZA solution contains 6 mg of liraglutide and the following inactive ingredients: disodium phosphate dihydrate, 1.42 mg; propylene glycol, 14 mg; phenol, 5.5 mg; and water for injection. VICTOZA has a pH of approximately 8.15, hydrochloric acid or sodium hydroxide may be added to adjust pH. Each pre-filled pen contains a 3 mL solution of VICTOZA equivalent to 18 mg liraglutide (free-base, anhydrous).
Mechanism of Action
Liraglutide is an acylated human Glucagon-Like Peptide-1 (GLP-1) receptor agonist with 97% amino acid sequence homology to endogenous human GLP-1(7-37). GLP-1(7-37) represents <20% of total circulating endogenous GLP-1. Like GLP-1(7-37), liraglutide activates the GLP-1 receptor, a membrane-bound cell-surface receptor coupled to adenylyl cyclase by the stimulatory G-protein, Gs, in pancreatic beta cells. Liraglutide increases intracellular cyclic AMP (cAMP) leading to insulin release in the presence of elevated glucose concentrations. This insulin secretion subsides as blood glucose concentrations decrease and approach euglycemia. Liraglutide also decreases glucagon secretion in a glucose-dependent manner. The mechanism of blood glucose lowering also involves a delay in gastric emptying.
GLP-1(7-37) has a half-life of 1.5-2 minutes due to degradation by the ubiquitous endogenous enzymes, dipeptidyl peptidase IV (DPP-IV) and neutral endopeptidases (NEP). Unlike native GLP-1, liraglutide is stable against metabolic degradation by both peptidases and has a plasma half-life of 13 hours after subcutaneous administration. The pharmacokinetic profile of liraglutide, which makes it suitable for once daily administration, is a result of self-association that delays absorption, plasma protein binding and stability against metabolic degradation by DPP-IV and NEP.
What is the most important information I should know about Victoza?
Victoza may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats and mice, Victoza and medicines that work like Victoza caused thyroid tumors, including thyroid cancer. It is not known if Victoza will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Do not use Victoza if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not use Victoza?
Do not use Victoza if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- you are allergic to liraglutide or any of the ingredients in Victoza. See the end of this Medication Guide for a complete list of ingredients in Victoza.
What should I tell my healthcare provider before using Victoza?
Before using Victoza, tell your healthcare provider if you have any other medical conditions, including if you:
- have or have had problems with your pancreas, kidneys, or liver.
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
- are pregnant or plan to become pregnant. It is not known if Victoza will harm your unborn baby. Tell your healthcare provider if you become pregnant while using Victoza.
- are breastfeeding or plan to breastfeed. It is not known if Victoza passes into your breast milk. You should talk with your healthcare provider about the best way to feed your baby while using Victoza.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Victoza may affect the way some medicines work and some medicines may affect the way Victoza works.
Before using Victoza, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes, including insulin or sulfonylureas.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Victoza?
- Read the Instructions for Use that come with Victoza.
- Use Victoza exactly as your healthcare provider tells you to.
- Your healthcare provider should show you how to use Victoza before you use it for the first time.
- Use Victoza 1 time each day, at any time of the day.
- Victoza may be taken with or without food.
- Victoza is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm. Do not inject Victoza into a muscle (intramuscularly) or vein (intravenously).
- Do not mix insulin and Victoza together in the same injection.
- You may give an injection of Victoza and insulin in the same body area (such as your stomach area), but not right next to each other.
- If you miss a dose of Victoza, take the missed dose at the next scheduled dose. Do not take 2 doses of Victoza at the same time.
- If you take too much Victoza, call your healthcare provider right away. Taking too much Victoza may cause severe nausea, severe vomiting, and low blood sugar (hypoglycemia).
- Change (rotate) your injection site with each injection. Do not use the same site for each injection.
- Do not share your Victoza pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- The Victoza pen you are using should be thrown away 30 days after you start using it.
Your dose of Victoza and other diabetes medicines may need to change because of:
- change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet or because of other medicines you take.
What are the possible side effects of Victoza?
Victoza may cause serious side effects, including:
- See “What is the most important information I should know about Victoza?”
- Inflammation of your pancreas (pancreatitis). Stop using Victoza and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Victoza with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. In children who are 10 years of age and older, the risk for low blood sugar may be higher with Victoza regardless of use with another medicine that can also lower blood sugar. Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion or drowsiness
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability, or mood changes
- hunger
- weakness
- feeling jittery
- Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse.
- Serious allergic reactions. Stop using Victoza and get medical help right away, if you have any symptoms of a serious allergic reaction including:
- swelling of your face, lips, tongue or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
- Gallbladder problems. Gallbladder problems have happened in some people who take Victoza. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in the right or middle upper stomach area
- nausea and vomiting
- fever
- your skin or the white part of your eyes turns yellow
The most common side effects of Victoza may include nausea, diarrhea, vomiting, decreased appetite, indigestion and constipation.
Talk to your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of Victoza.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of Victoza
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Victoza for a condition for which it was not prescribed. Do not give Victoza to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Victoza that is written for health professionals.
How should I store Victoza?
Before use:
- Store your new, unused Victoza pen in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC).
- If Victoza is stored outside of refrigeration (by mistake) prior to first use, it should be used or thrown away within 30 days.
- Do not freeze Victoza or use Victoza if it has been frozen. Do not store Victoza near the refrigerator cooling element.
Pen in use:
- Use a Victoza pen for only 30 days. Throw away a used Victoza pen 30 days after you start using it, even if some medicine is left in the pen.
- Store your Victoza pen at 59ºF to 86ºF (15ºC to 30ºC), or in a refrigerator at 36ºF to 46ºF (2°C to 8°C).
- When carrying the pen away from home, store the pen at a temperature between 59ºF to 86ºF (15ºC to 30ºC).
- If Victoza has been exposed to temperatures above 86ºF (30°C), it should be thrown away.
- Protect your Victoza pen from heat and sunlight.
- Keep the pen cap on when your Victoza pen is not in use.
- Always remove the needle after each injection and store your pen without the needle attached. This reduces the risk of contamination, infection, leakage and inaccurate dosing.
What are the ingredients in Victoza?
Active Ingredient: liraglutide
Inactive Ingredients: disodium phosphate dihydrate, propylene glycol, phenol and water for injection
For more information, go to victoza.com or call 1-877-484-2869.
Instructions for use for Victoza
Victoza (liraglutide) injection

First read the Medication Guide that comes with your Victoza pen and then read this Patient Instructions for Use for information about how to use your Victoza pen the right way.
These instructions do not take the place of talking with your healthcare provider about your medical condition or your treatment.
Do not share your Victoza Pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Your Victoza pen contains 3 mL of Victoza and will deliver doses of 0.6 mg, 1.2 mg or 1.8 mg. The number of doses that you can take with a Victoza pen depends on the dose of medicine that is prescribed for you. Your healthcare provider will tell you how much Victoza to take.
Victoza pen should be used with Novo Nordisk disposable needles. Talk to your healthcare provider or pharmacist for more information about needles for your Victoza pen.
Important Information
Δ Always use a new needle for each injection to prevent contamination.
Δ Always remove the needle after each injection, and store your pen without the needle attached. This reduces the risk of contamination, infection, leakage of liraglutide, blocked needles and inaccurate dosing.
Δ Keep your Victoza pen and all medicines out of the reach of children.
Δ If you drop your Victoza pen, repeat “First Time Use For Each New Pen” (Steps A through D).
Δ Be careful not to bend or damage the needle.
Δ Do not use the cartridge scale to measure how much Victoza to inject.
Δ Be careful when handling used needles to avoid needle stick injuries.
Δ You can use your Victoza pen for up to 30 days after you use it the first time.
First Time Use for Each New Pen
Step A. Check the Pen
- Take your new Victoza pen out of the refrigerator.
- Wash hands with soap and water before use.
- Check pen label before each use to make sure it is your Victoza pen.
- Pull off pen cap (See Figure A).
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- Wipe the rubber stopper with an alcohol swab.

Step B. Attach the Needle
- Remove protective tab from outer needle cap (See Figure B).
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure.

- Pull off outer needle cap (See Figure C). Do not throw away.
- Pull off inner needle cap and throw away (See Figure D). A small drop of liquid may appear. This is normal.

Step C. Dial to the Flow Check Symbol
This Step is done only Once for each new pen and is Only required the first time you use a new pen.
- Turn dose selector until flow check symbol (–) lines up with pointer (See Figure E). The flow check symbol does not administer the dose as prescribed by your healthcare provider.
- To select the dose prescribed by your healthcare provider, continue to Step G under “Routine Use”.

Step D. Prepare the Pen
- Hold pen with needle pointing up.
- Tap cartridge gently with your finger a few times to bring any air bubbles to the top of the cartridge (See Figure F).

- Keep needle pointing up and press dose button until 0 mg lines up with pointer (See Figure G). Repeat Steps C and D, up to 6 times, until a drop of Victoza appears at the needle tip.
If you still see no drop of Victoza, use a new pen and contact Novo Nordisk at 1-877-484-2869.
Continue to Step G under “Routine Use” →

Routine Use
Step E. Check the Pen
- Take your Victoza pen from where it is stored.
- Wash hands with soap and water before use.
- Check pen label before each use to make sure it is your Victoza pen.
- Pull off pen cap (See Figure H).

- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- Wipe the rubber stopper with an alcohol swab.
Step F. Attach the Needle
- Remove protective tab from outer needle cap.
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure (See Figure I).

- Pull off outer needle cap. Do not throw away (See Figure J).
- Pull off inner needle cap and throw away (See Figure K). A small drop of liquid may appear. This is normal.

Step G. Dial the Dose
- Victoza pen can give a dose of 0.6 mg (starting dose), 1.2 mg or 1.8 mg. Be sure that you know the dose of Victoza that is prescribed for you.
- Turn the dose selector until your needed dose lines up with the pointer (0.6 mg, 1.2 mg or 1.8 mg) (See Figure L).

- You will hear a “click” every time you turn the dose selector. Do not set the dose by counting the number of clicks you hear.
- If you select a wrong dose, change it by turning the dose selector backwards or forwards until the correct dose lines up with the pointer. Be careful not to press the dose button when turning the dose selector. This may cause Victoza to come out.
Step H. Injecting the Dose
- Insert needle into your skin in the stomach (abdomen), thigh or upper arm. Use the injection technique shown to you by your healthcare provider. Do not inject Victoza into a vein or muscle.
- Press down on the center of the dose button to inject until 0 mg lines up with the pointer (See Figure M).

- Be careful not to touch the dose display with your other fingers. This may block the injection.
- Keep the dose button pressed down and make sure that you keep the needle under the skin for a full count of 6 seconds to make sure the full dose is injected. Keep your thumb on the injection button until you remove the needle from your skin (See Figure N).

- Change (rotate) your injection sites within the area you choose for each dose. Do not use the same injection site for each injection.
Step I. Withdraw Needle
- You may see a drop of Victoza at the needle tip. This is normal and it does not affect the dose you just received. If blood appears after you take the needle out of your skin, apply light pressure, but do not rub the area (See Figure O).

Step J. Remove and Dispose of the Needle
- Carefully put the outer needle cap over the needle (See Figure P). Unscrew the needle

- Safely remove the needle from your Victoza pen after each use.
- Put your used Victoza pen and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: HTTP://WWW.FDA.GOV/SAFESHARPSDISPOSAL.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Caring for your Victoza pen
- After removing the needle, put the pen cap on your Victoza pen and store your Victoza pen without the needle attached (See Figure Q).

- Do not try to refill your Victoza pen – it is prefilled and is disposable.
- Do not try to repair your pen or pull it apart.
- Keep your Victoza pen away from dust, dirt and liquids.
- If cleaning is needed, wipe the outside of the pen with a clean, damp cloth.
How should I store Victoza?
Before use:
- Store your new, unused Victoza pen in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC).
- If Victoza is stored outside of refrigeration (by mistake) prior to first use, it should be used or thrown away within 30 days.
- Do not freeze Victoza or use Victoza if it has been frozen. Do not store Victoza near the refrigerator cooling element.
Pen in use:
- Use a Victoza pen for only 30 days. Throw away a used Victoza pen 30 days after you start using it, even if some medicine is left in the pen.
- Store your Victoza pen at 59ºF to 86ºF (15ºC to 30ºC), or in a refrigerator at 36ºF to 46ºF (2°C to 8°C).
- When carrying the pen away from home, store the pen at a temperature between 59ºF to 86ºF (15ºC to 30ºC).
- If Victoza has been exposed to temperatures above 86ºF (30°C), it should be thrown away.
- Protect your Victoza pen from heat and sunlight.
- Keep the pen cap on when your Victoza pen is not in use.
- Always remove the needle after each injection and store your pen without the needle attached. This reduces the risk of contamination, infection, leakage and inaccurate dosing.
Label
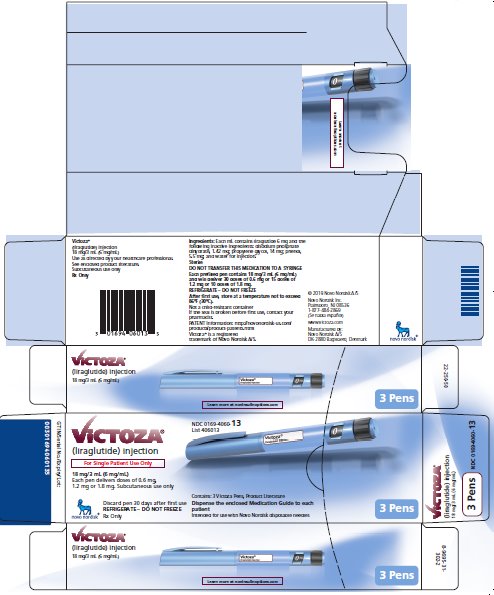
PRINCIPAL DISPLAY PANEL – 3 VICTOZA PENS
- NDC 0169-4060-13
- List 406013
- VICTOZA®
- (liraglutide) injection
- For Single Patient Use Only
- 18 mg/3 mL (6 mg/mL)
- Each pen delivers doses of 0.6 mg, 1.2 mg or 1.8 mg
- Subcutaneous use only
- Discard pen 30 days after first use
- REFRIGERATE – DO NOT FREEZE
- Rx Only
- Contains: 3 Victoza Pens, Product Literature
- Dispense the enclosed Medication Guide to each patient
- Intended for use with Novo Nordisk disposable needles
- 3 Pens
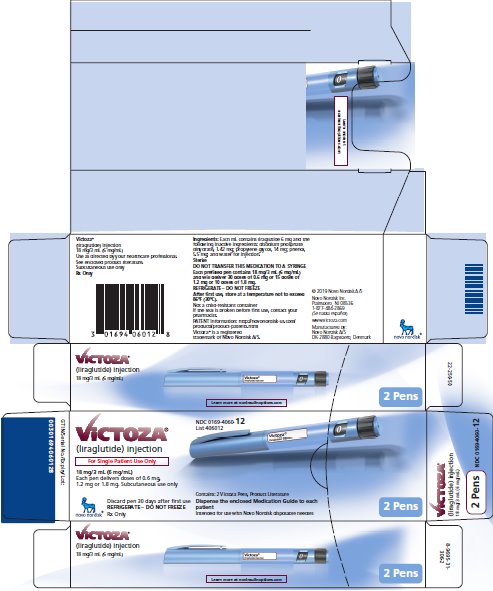
PRINCIPAL DISPLAY PANEL – 2 VICTOZA PENS
- NDC 0169-4060-12
- List 406012
- VICTOZA®
- (liraglutide) injection
- For Single Patient Use Only
- 18 mg/3 mL (6 mg/mL)
- Each pen delivers doses of 0.6 mg, 1.2 mg or 1.8 mg
- Subcutaneous use only
- Discard pen 30 days after first use
- REFRIGERATE – DO NOT FREEZE
- Rx Only
- Contains: 2 Victoza Pens, Product Literature
- Dispense the enclosed Medication Guide to each patient
- Intended for use with Novo Nordisk disposable needles
- 2 Pens
SRC: NLM .